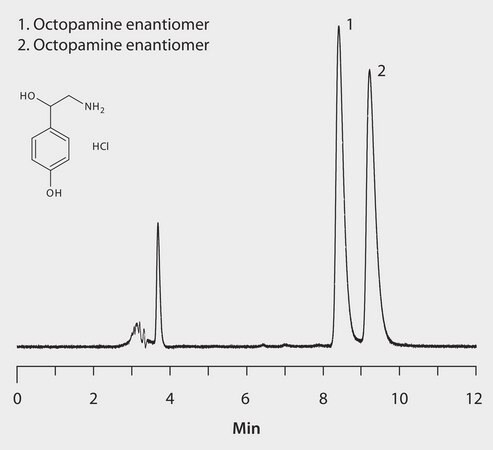
HPLC Analysis of Octopamine Enantiomers on LARIHC™ CF6-P
Matériaux
Étalon
CONDITIONS
column
LARIHC CF6-P, 25 cm x 4.6 mm I.D., 5 μm particles (AZYP Part No.L1001, available from Supelco/Sigma-Aldrich as a custom item.)
mobile phase
[A] methanol: [B] acetonitrile: [C] acetic acid: [D] triethylamine, (70:30:0.3.0.2, A:B:C:D)
flow rate
1 mL/min
column temp.
20 °C
detector
UV, 254 nm
injection
5 μL
sample
Octopamine, 0.3 mg/mL in ethanol
Description
Description générale
Cyclofructans are cyclic oligosaccharides and the newest class of chiral stationary phases for HPLC, SFC, and HILIC. Invented by Prof. Daniel W. Armstrong and introduced by AZYP, LLC, the LARICH and FRULIC derivatized cyclofructan-based columns are now available through Supelco/Sigma-Aldrich. The LARIHC CF6-P chiral stationary phase (CSP) was developed as an alkyl derivatized cyclofructan 6 chiral stationary phase. It has demonstrated pronounced enantioselectivity toward all types of primary amines, such as amino alcohols, amino esters, and amino amides. Baseline separation was achieved for simple aliphatic racemic amines that contained no other functionality. Unlike all current crown ether chiral stationary phases, this new column works more effectively with organic solvents and supercritical fluids. It also appears to have great capabilities for preparative-scale separations. No other existing phase can efficiently separate primary amines as well as the LARIHC CF6-P phase. A recent study showed that this phase alone can separate 93% of tested racemic primary amines.
Informations légales
LARIHC is a trademark of AZYP, LLC