This product is freely soluble in ethanol. No recommended dosage for cells is available.
1642802
USP
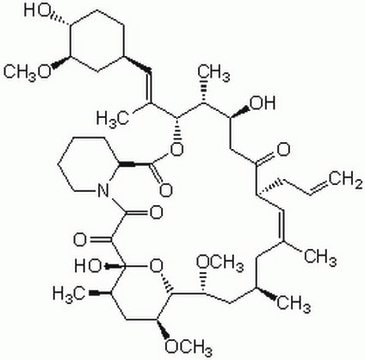
Tacrolimus
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
FK-506 monohydrate, Tacrolimus
Sélectionner une taille de conditionnement
Sélectionner une taille de conditionnement
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
tacrolimus
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
O.CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]2OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@]4(O)O[C@H]([C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)[C@H](C[C@H]4C)OC
InChI
1S/C44H69NO12.H2O/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7;/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3;1H2/b25-19+,27-21+;/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+;/m0./s1
Clé InChI
NWJQLQGQZSIBAF-MLAUYUEBSA-N
Informations sur le gène
human ... FKBP1A(2280)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Tacrolimus Capsules
- Tacrolimus Compounded Oral Suspension
Actions biochimiques/physiologiques
Remarque sur l'analyse
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
-
How do I reconstitute this product (100mg), and what is the recommended dosage to treat 1x10^6 cells (human PBMC) with this product?
1 answer-
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique