H3648
(±)-3-Hydroxydecanoic acid
≥98%
Synonyme(s) :
DL-β-Hydroxycapric acid
About This Item
Produits recommandés
Pureté
≥98%
Forme
powder
Groupe fonctionnel
carboxylic acid
Type de lipide
saturated FAs
Conditions d'expédition
ambient
Température de stockage
2-8°C
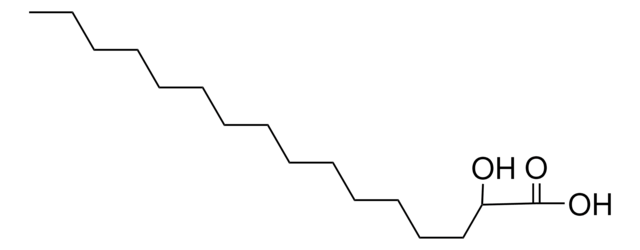
Chaîne SMILES
CCCCCCCC(O)CC(O)=O
InChI
1S/C10H20O3/c1-2-3-4-5-6-7-9(11)8-10(12)13/h9,11H,2-8H2,1H3,(H,12,13)
Clé InChI
FYSSBMZUBSBFJL-UHFFFAOYSA-N
Application
- LORE receptor homomerization is required for 3-hydroxydecanoic acid-induced immune signaling and determines the natural variation of immunosensitivity within the Arabidopsis genus.: This study unveils the crucial role of 3-Hydroxydecanoic acid in mediating immune responses through LORE receptor homomerization in plants, providing insights into the molecular mechanisms of plant defense and potential agricultural applications (Eschrig et al., 2024).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)