158240
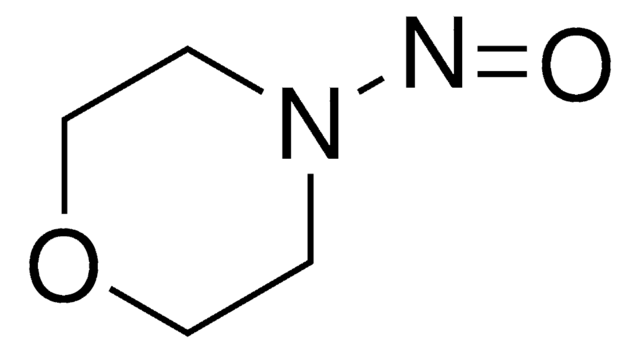
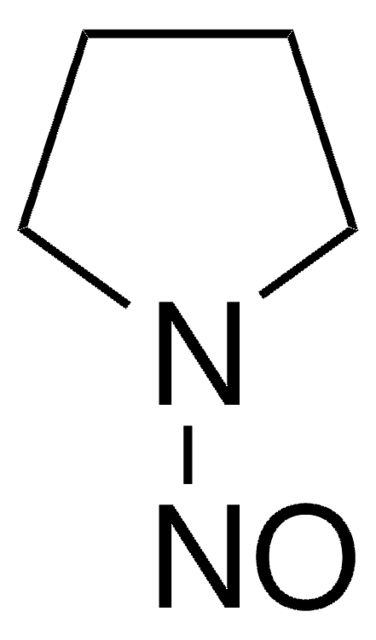
1-Nitrosopyrrolidine
99%
Synonyme(s) :
N-Nitrosopyrrolidine, NPYR
About This Item
Produits recommandés
Pureté
99%
Indice de réfraction
n20/D 1.489 (lit.)
Point d'ébullition
214 °C (lit.)
Densité
1.085 g/mL at 25 °C (lit.)
Chaîne SMILES
O=NN1CCCC1
InChI
1S/C4H8N2O/c7-5-6-3-1-2-4-6/h1-4H2
Clé InChI
WNYADZVDBIBLJJ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Carc. 2
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
208.4 °F - closed cup
Point d'éclair (°C)
98 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Cancer research has revealed that the classical model of carcinogenesis, a three step process consisting of initiation, promotion, and progression, is not complete.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique