909564
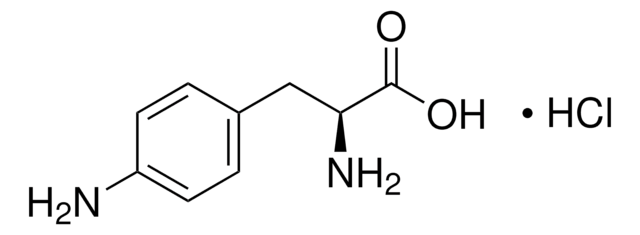
4-Azido-L-phenylalanine
≥98%
Synonyme(s) :
3-(p-Azidophenyl)-L-alanine hydrochloride, 4-Azidophenylalanine hydrochloride, 4-N3-L-Phenylalanine, p-Azido-L-phenylalanine hydrochloride, p-Azido-Phe*HCl, p-Azidophenylalanine hydrochloride, H-4-Azido-Phe*HCl, H-L-4-Azido-Phe-OH, H-L-Phe(4-N3)-OH, H-Phe(4-N3)*HCl, Phe(pN3)*HCl
About This Item
Produits recommandés
Pureté
≥98%
Forme
powder
Capacité de réaction
reaction type: click chemistry
reaction type: solution phase peptide synthesis
Disponibilité
available only in USA
Température de stockage
−20°C
InChI
1S/C9H10N4O2/c10-8(9(14)15)5-6-1-3-7(4-2-6)12-13-11/h1-4,8H,5,10H2,(H,14,15)/t8-/m0/s1
Clé InChI
NEMHIKRLROONTL-QMMMGPOBSA-N
Application
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Self-react. C
Code de la classe de stockage
5.2 - Organic peroxides and self-reacting hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Certificats d'analyse (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique