45460
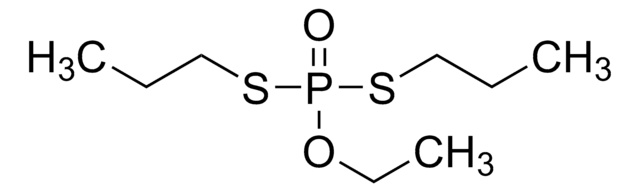
Disulfoton
PESTANAL®, analytical standard
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Gamme de produits
PESTANAL®
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
agriculture
environmental
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
CCOP(=S)(OCC)SCCSCC
InChI
1S/C8H19O2PS3/c1-4-9-11(12,10-5-2)14-8-7-13-6-3/h4-8H2,1-3H3
Clé InChI
DOFZAZXDOSGAJZ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
271.4 °F
Point d'éclair (°C)
133 °C
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique