16413
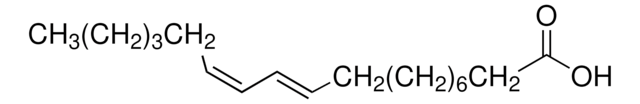
(9Z,11E)-9,11-Octadecadienoic acid
analytical standard
Synonyme(s) :
(9Z,11E)-9,11-Octadecadienoic acid, 9Z,11E-CLA, Bovinic acid, Linoleic acid (9-cis, 11-trans)
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Qualité
analytical standard
Pureté
≥96.0% (HPLC)
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Indice de réfraction
n20/D 1.478-1.483
Format
neat
Groupe fonctionnel
carboxylic acid
Température de stockage
−20°C
Chaîne SMILES
CCCCCC\C=C\C=C/CCCCCCCC(O)=O
InChI
1S/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h7-10H,2-6,11-17H2,1H3,(H,19,20)/b8-7+,10-9-
Clé InChI
JBYXPOFIGCOSSB-GOJKSUSPSA-N
Description générale
Application
Conditionnement
Produits recommandés
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique