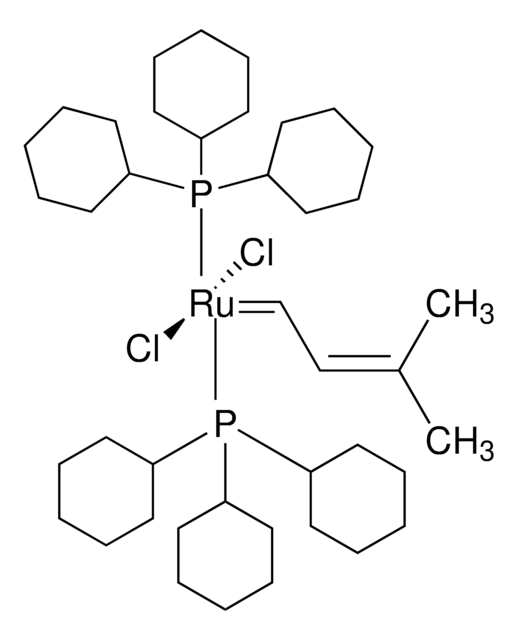
682365
[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(3-methyl-2-butenylidene)(tricyclohexylphosphine)ruthenium(II)
Umicore
Synonyme(s) :
Isopentenylidene(1,3-dimesitylimidazolidin-2-ylidene)(tricyclohexylphosphine)ruthenium(II) dichloride
About This Item
Produits recommandés
Pertinence de la réaction
core: ruthenium
reagent type: catalyst
reaction type: Olefin Metathesis
Température de stockage
2-8°C
Chaîne SMILES
C1CCC(CC1)P(C2CCCCC2)C3CCCCC3.C\C(C)=C/C=[Ru](Cl)(Cl)=C4N(CCN4c5c(C)cc(C)cc5C)c6c(C)cc(C)cc6C
InChI
1S/C21H26N2.C18H33P.C5H8.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-4-5(2)3;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1,4H,2-3H3;2*1H;/q;;;;;+2/p-2
Clé InChI
LCOFYVWULBZOTA-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Autres remarques
Informations légales
Product License
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Flam. Sol. 2
Code de la classe de stockage
4.1B - Flammable solid hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Contenu apparenté
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique



![[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(benzylidene)bis(3-bromopyridine)ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/261/898/e64eea4e-5a09-4c7d-b400-c43b3517de2a/640/e64eea4e-5a09-4c7d-b400-c43b3517de2a.png)

![[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro[3-(2-pyridinyl-κN)propylidene-κC]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/416/586/0907c050-f5d7-4a53-b22f-23170c2703c2/640/0907c050-f5d7-4a53-b22f-23170c2703c2.png)