All Photos(1)
About This Item
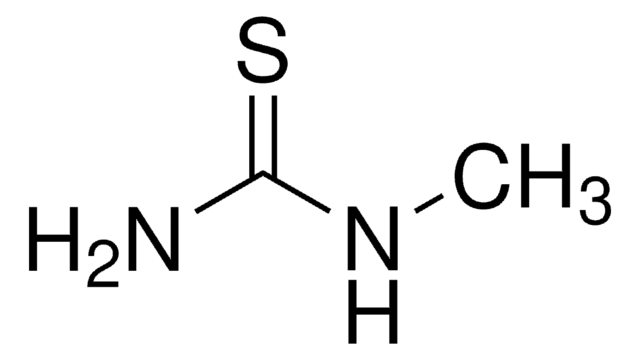
Linear Formula:
CH3CONHCSNH2
CAS Number:
Molecular Weight:
118.16
Beilstein:
969960
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
165-169 °C (lit.)
SMILES string
CC(=O)NC(N)=S
InChI
1S/C3H6N2OS/c1-2(6)5-3(4)7/h1H3,(H3,4,5,6,7)
InChI key
IPCRBOOJBPETMF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Olufunke Olagunju et al.
The journal of physical chemistry. A, 110(7), 2396-2410 (2006-02-17)
The oxidation reactions of N-acetylthiourea (ACTU) by chlorite and chlorine dioxide were studied in slightly acidic media. The ACTU-ClO(2)(-) reaction has a complex dependence on acid with acid catalysis in pH > 2 followed by acid retardation in higher acid
Chunlian Qin et al.
Talanta, 199, 131-139 (2019-04-07)
Detection and identification of bitter compounds draw great attention in pharmaceutical and food industry. Several well-known agonists of specific bitter taste receptors have been found to exhibit anti-cancer effects. For example, N-C=S-containing compounds, such as allyl-isothiocyanates, have shown cancer chemo-preventive
László Somogyi et al.
Carbohydrate research, 344(1), 134-135 (2008-11-04)
Treatment with concd HCl/MeOH transformed N-(tetra-O-acetyl-beta-D-glucopyranosyl)-N'-acetylthiourea, via selective cleavage of the primary alcoholic ester group, into the title compound.
V S Gudumak et al.
Laboratornoe delo, (9)(9), 15-18 (1989-01-01)
The authors have examined the possibility of replacing sodium arsenite with acetyl thiourea and of using butanol acidified with phosphoric acid in measuring sialic acids in biologic material. The results evidence a sufficient specificity, reproducibility, higher sensitivity and lower toxicity
Raushan K Singh et al.
Bioorganic & medicinal chemistry letters, 21(19), 5920-5923 (2011-08-26)
We report, for the first time, that certain N-acetylthiourea derivatives serve as highly potent and isozyme selective activators for the recombinant form of human histone deacetylase-8 in the assay system containing Fluor-de-Lys as a fluorescent substrate. The experimental data reveals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service