Analysis of Antibiotics— Tetracyclines, Sulfonamides and Quinolones Residues in Pork Meat by LC-MS acc. to GB 31658.17-2021
Jack Wang
R&D APAC lab, Shanghai, China
Abstract
A sensitive liquid chromatography-mass spectrometry method was developed for the determination of antibiotic drug residues in pork meat in accordance with the GB 31658.17-2021. Target analytes included 19 sulfonamides, 13 quinolones and three tetracycline drugs. Samples were extracted with Mcllvaine Na2EDTA buffer solution, purified by solid-phase extraction using a modern HLB phase (Supel™ Swift HLB), analyzed by liquid chromatography-tandem mass spectrometry employing a Purospher® STAR RP-18e UHPLC column, and quantified by the external standard method. The developed method complied with the performance criteria required by the GB 31658.17-2021.
Section Overview
Introduction
The widespread use of antibiotics in animal husbandry and their associated residues in animal meat has been a worldwide concern, as they are known to have detrimental health effects for the consumers.1,2 Sulfonamides, quinolones, and tetracycline antibiotics are widely used in livestock and poultry breeding. There is therefore a need to monitor these residues in meat. As these veterinary drugs are often used at random, the analytical challenge is the simultaneous detection of multiple antibiotic classes within the same sample. At present, HPLC and LC-MS/MS methods are mainly used for the analysis and detection of antibiotics, whereas LC-MS/MS is the main analysis and detection technique.3,4
Many national and international authorities and limits and issued detection methods for various matrices. For China, the GB 31658.17-2021 method5 specifies the type of substrate for testing samples as: muscle, liver and kidney tissue of cattle, such as sheep, pigs, and chicken. The method describes the residue determination of 19 sulfonamides, 13 quinolones, and 3 tetracyclines in the aforementioned matrices. The samples are extracted with Mcllvaine-Na2EDTA buffer solution, then purified by solid-phase extraction (SPE) using a hydrophilic lipophilic balanced (HLB) SPE phase, and the target compounds are determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an external matrix-matched calibration for quantification.
As performance & quality control criteria, the GB 31658.17-2021 defines requirements as follows:
- Limits of Detection (LOD) and Limits of Quantification (LOQ) with LOD £5 μg/kg and LOQ £15 μg/kg.
- Recovery at the addition concentration level of 10-500 μg/kg to be in the range of 60%-110%.
- The relative standard deviation (%RSD) of the precision test should be less than 15%.
For the here described application, the sample pre-treatment was carried out in accordance with the GB 31658.17-2021 standard using a Supel™ Swift HLB SPE tube. 19 sulfonamides, 13 quinolones, and 3 tetracyclines (Table 2) were analyzed and quantified against a matrix match calibration by LC-MS/MS using a Purospher® STAR RP-18 endcapped (2 µm) UHPLC column (Table 1). The performance was compared against the quality control requirements of the GB 31658.17-2021 standard.
Experimental
Sample Preparation
500 g of fresh pork meat was homogenized and sampled by the quarter method into polyethylene bottles.
Reagent Preparation
- Sodium dihydrogen phosphate solution (0.05 mol/L): 7.8 g sodium dihydrogen phosphate dihydrate in 1000 mL of water.
- Disodium hydrogen phosphate solution (0.05 mol/L): 17.9 g disodium hydrogen phosphate dodecahydrate in 1000 mL of water.
- Phosphate buffer solution: 190 mL of 0.05 mol/L sodium dihydrogen phosphate solution top up to 1000 mL with 0.05 mol/L disodium hydrogen phosphate solution.
- Sodium hydroxide solution (1 mol/L): 4 g sodium hydroxide in 100 mL of water.
- Sodium hydroxide solution (0.03 mol/L): 3 mL of 1 mol/L sodium hydroxide solution topped up to 100 mL with water.
- Mcllvaine-Na2EDTA buffer solution: 12.9 g citric acid, 10.9 g disodium hydrogen phosphate, and 39.2 g disodium ethylenediamine tetraacetate were added to 900 mL water and adjusted to pH 5.0 ± 0.2 with 1 mol/L sodium hydroxide solution before topping up to 1000 mL with water.
- Eluent (SPE): To 150 mL methanol, add 150 mL ethyl acetate, 6 mL ammonia, and mix well.
- Complex solution (reconstitution mix): To 40 mL water, add 5 mL methanol, 5 mL acetonitrile, and 0.05 mL formic acid and mix well.
Standard Preparation
- Stock solution I (1 mg/mL): Take 10.0 mg of each compound (sulfonamides, quinolones, and tetracycline) in separate 10 mL volumetric flasks. Tetracyclines and sulfonamides were dissolved with methanol and topped to 10 mL. Quinolones were dissolved in 0.03 mol/L sodium hydroxide solution and topped to 10 mL. All solutions were stored below -18°C and to be used within 6 months.
- Stock solution II (10 µg/mL): Take 0.1 mL of each Stock solution I in separate 10 mL volumetric flasks and top each to mark with methanol. All solutions were stored below -18°C and to be used within 1 month.
- Stock solution III (1 µg/mL): Take 1 mL of each Stock solution II in separate 10 mL volumetric flasks and top each to mark with methanol. All solutions were stored below -18°C and to be used within 1 month.
- Calibration standard solutions 1-5: Take 2, 10, 50, 100, and 500 μL of each Stock solution III in a 1 mL volumetric flask and top to mark with methanol for 5 working standard solutions. The concentrations of the drugs are 2.0, 10.0, 50.0, 100, and 500 μg/L.
Sample Preparation
- Extraction: To 1 g of the homogenized pork sample, add 8 mL Mcllvaine-Na2EDTA buffer. Vortex for 1 min followed by sonication for 20 min before centrifugation at 10,000 rpm at -2°C for 5 min. Recover the supernatant and set aside. Repeat extraction twice and pool the supernatant as a combined extract.
- Solid phase extraction (SPE): Condition and equilibrate the Supel™ Swift HLB SPE Tubes (200 mg/6 mL) tube with 5 mL methanol and 5 mL water. Load the entire pooled supernatant onto the column. Wash the SPE tube with 5 mL of water and 5 mL of 20% v/v methanol. Elute the analytes with 10 mL eluent solution. Collect the eluate and evaporate under nitrogen at 45°C in a water bath. Add 1 mL of the complex solution to reconstitute the residue by swirling for 1 min. Centrifuge at 14,000 rpm for 5 min before filtering through a 0.22 μm PES syringe filter (e.g., Millex®) for LC-MS/MS analysis.
- Spiking experiments: Add 50 and 100 μL each of Stock solutions III to 1.0 g pork samples. The resulting spike concentrations of 50 and 100 µg/kg are used for the determination of precision and recovery, respectively.
LC-MS/MS Analysis
The LC-MS/MS analysis of the external calibration standards and the meat extracts was performed with the conditions described in Table 1.
Results & Discussion
According to the requirement of the GB method, a gradient elution and detection by multi reaction monitoring (MRM) was used for the LC-MS analysis (Tables 1 & 2). The separation of a 10 µg/L standard solution is displayed in Figure 1 and shows sufficient resolution for the analysis by LC-MS/MS using TIC-MRM in less than 12 min.
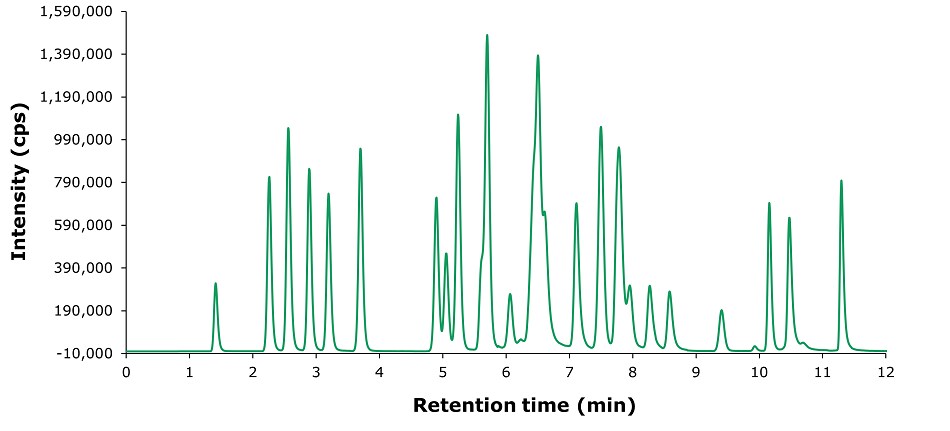
Figure 1.Chromatogram (TIC-MRM traces) of a standards solution at 10 μg/L in methanol.
Calibration
Tetracyclines, sulfonamides and quinolones were externally calibrated (matrix standard solution), and five standard solutions were prepared using a blank pork sample extract. solutions in the range of 2-500 µg/L. The linearity for the 35 compounds showed R2 values between 0.9947 and 0.9998. As an example, in Table 3 and Figure 2, the calibration data for sulfacetamide is shown.
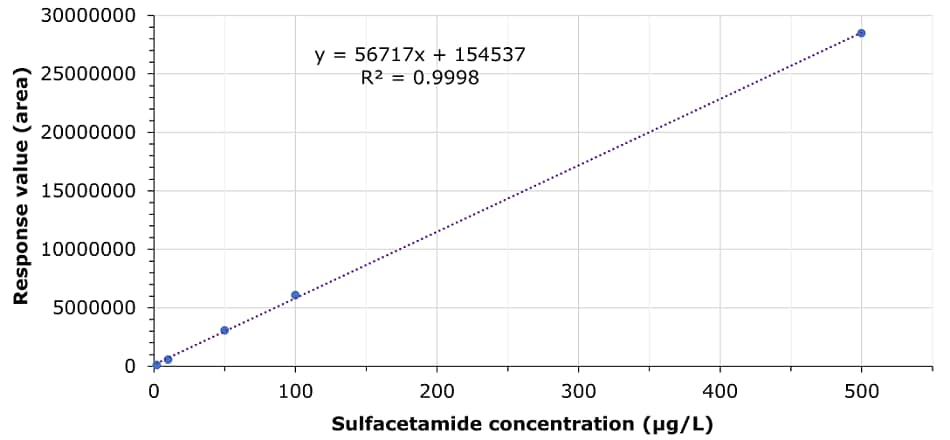
Figure 2.Calibration curve for sulfacetamide 2, 10, 50, 100 and 500 μg/L.
Precision and Recovery (%)
The method’s precision was determined by spiked samples at 50 µg/kg and showed %RSDs in the range of 1.0 and 3.5% (n=7) and by that complying with the <15% criteria of the GB method.
The recovery was investigated using samples spiked at 100 µg/kg and showed average recoveries (n=6) between 76.9% and 102%, meeting the 60-110% requirement of the official method.
In Tables 4 & 5 are example data sets for precision assessments and recovery determination for the 3 compounds shown.
Sensitivity
According to the specifications set in the GB31658.17-2021 standard for the determination of Limit of Detection (LOD) & Limit of Quantification (LOQ), this study used the extract of a blank pork sample, where 3N/X was used to determine LOD, 10N/X was used to determine LOQ (Table 6). The GB method states requirements for sensitivity of LOD ≤ μg/kg and LOQ ≤ μg/kg, hence the here-shown method complies with this criteria.

Figure 3.Chromatogram (TIC-MRM) of pork samples spiked with 10 µg/kg of tetracyclines, sulfonamides and quinolones.

Figure 4.Chromatogram (TIC-MRM) of blank pork sample.
Conclusion
35 antibiotic drug residues from tetracyclines, sulfonamides, and quinolones families were analyzed following the GB 31658.17-2021 method. For the SPE cleanup, a Supel™ Swift HLB tube and for the LC-MS/MS analysis a Purospher® STAR RP-18 endcapped (2 µm) UHPLC column were used. The external calibration for the analytes was linear in the range of 2 to 500 μg/L with a correlation coefficient R2 >0.995. The method’s precision was determined by spiked samples at 50 µg/kg and showed % RSDs in the range of 1.0 and 3.5% (n=7). The recovery was investigated using samples spiked at 100 µg/kg and showed average recoveries (n=6) between 76.9% and 102%. The LODs and LOQs for an actual sample were determined according to the GB method with 3.0 μg/kg and 10.0 μg/kg, respectively.
The results of the here-shown method comply with all the criteria set out in the GB standard regarding precision, recovery, and sensitivity (LOD & LOQ). Therefore, the Supel™ Swift HLB SPE tube and the Purospher® STAR RP-18e UHPLC column can be used for the determination of antibiotic residues in food samples according to the GB 31658.17-2021 standard.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?