Dudley Reagents
Introduction
Benzyl and p-methoxy benzyl protecting groups are ubiquitous in organic synthesis; however, these protecting groups commonly require using acidic or basic reaction media and or otherwise non-mild reaction conditions, which sometimes are not compatible with other functional groups in the molecule. Dudley and coworkers have developed two reagents for the protection of alcohols and carboxylic acids under relatively neutral reaction conditions: benzyloxypyridinium triflate (Product No. 679674) and 2-(4-methoxybenzyloxy)-4-methylquinoline (Product No. 701440) for the protection of alcohols and carboxylic acids and alcohols, respectively.
Representative Applications
Benzyl-Protection of Alcohols and Carboxylic Acids
Typical procedures for the benzyl-protection of alcohols involve the use of a benzyl halide in combination with a base or benzyl trichloroacetimidate in combination with an acid. A neutral approach to this important transformation was reported by Dudley and co-workers using benzyloxypyridinium triflate, which is prepared in two steps from 2-chloropyridine. A variety of alcohols (Table 1) are protected under these reaction conditions, including primary, secondary, and tertiary alcohols, resulting in conversion to the protected derivative under relatively mild and neutral reaction conditions. Additionally, compounds containing stereogenic centers did not epimerize under the reaction conditions (Table 1, entry 4).1
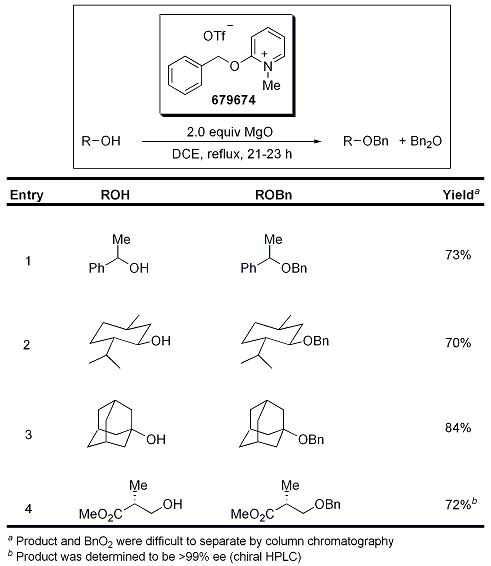
Table 1.Protection of Alcohols Using 679674
The use of benzyloxypyridinium triflate was also investigated in the selective protection of carboxylic acids to their analogous benzylic esters in the presence of Et3N, which the authors propose is used as a promoter and a scavenger. Several functionalized carboxylic acids were effectively converted to their benzyl ester counterparts, including alkyl, vinyl, alkynyl, and aryl, as well as selective conversions of derivatives containing acetate ester, aryl methyl ether, and free phenol functionalities (Table 2).2
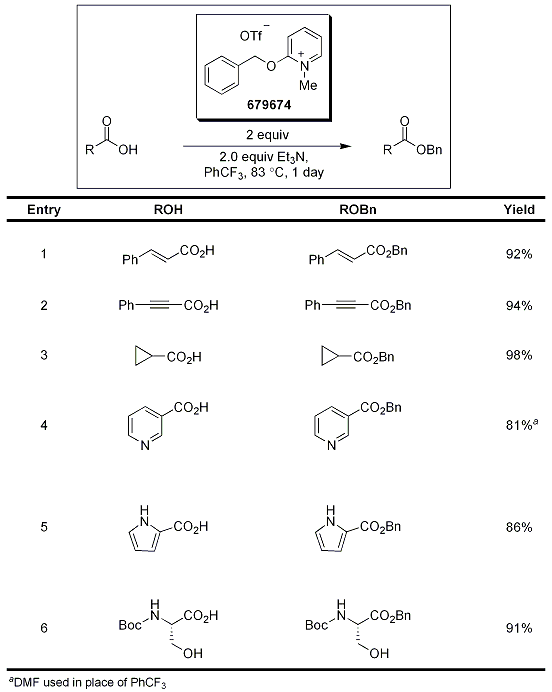
Table 2.Protection of Carboxylic Acids Using 679674
The authors propose the mechanism of the reaction (Scheme 1) to involve formation of the benzyl cation 1 under thermal conditions. The benzyl cation quickly reacts with the carboxylate complex 2. Remaining cation 1 reacts with excess Et3N before it can react with other functionalities present in the molecule and or before it can react with water.
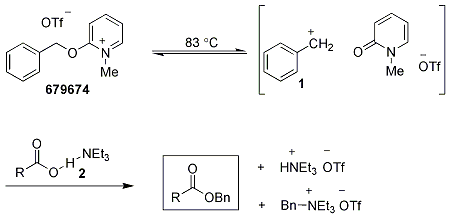
Scheme 1.Mechanism of Protection of Carboxylic Acids Using 679674
para-Methoxy Benzyl Protection of Alcohols
Protection with the para-methoxy benzyl protecting group (PMB) has been an ongoing challenge in organic chemistry due to the limitations with regard to the reagents that can be used. Common reagents (Williamson and trichloroacetimidate coupling reactions) typically require acidic or basic reaction media and present challenges with respect to long-term storage. After the use of benzyloxypyridinium triflate for the protection of alcohols was reported, the analogous PMB salt was prepared; however, the salt was not soluble in the most effective solvents for the transformation, such as trifluorotoluene and toluene. Therefore, the analogous quinoline salt was prepared, and while solubility was no longer a problem, it was unstable at room temperature. Therefore, the lepidine ether below (2-(4-methoxybenzyloxy)-4-methylquinoline) was prepared without salt formation and was used in combination with MeOTf to afford an active reagent in situ. The active reagent is more stable than other PMB transfer reagents such as PMB chloride and PMB trichloroacetimidate. A plethora of alcohols were protected under these reaction conditions, including primary, secondary, and tertiary. Additionally, challenging substrates such as cholesterol and substrates containing acid- or base-sensitive functionalities are readily protected under neutral reaction conditions. Additionally, 2-(4-methoxybenzyloxy)-4-methylquinoline is stable, and byproducts generated by its use are easily removed through aqueous workup or chromatography.3 The authors propose an SN1-type mechanism for the protection and it is imperative that the substrate does not react with MeOTf, but rather with the cation generated from the active reagent that is prepared in situ.
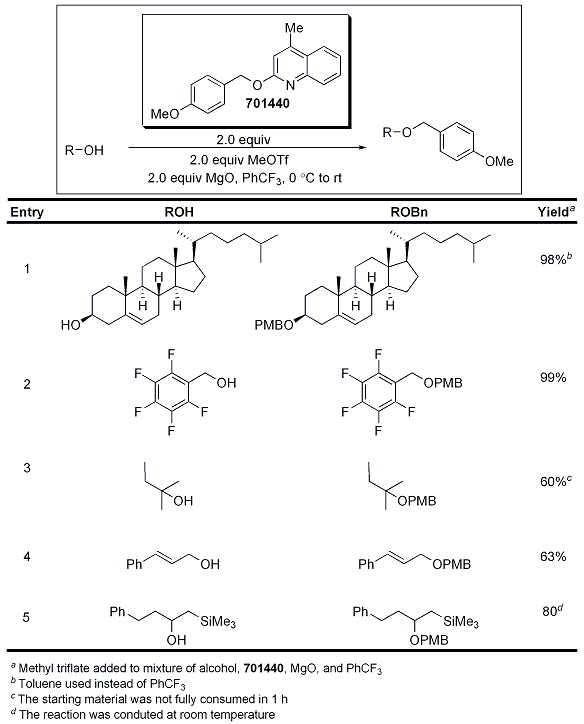
Table 3.Protection of Alcohols using 701440
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?