BINAP/SEGPHOS® Ligands and Complexes
William Sommer, Daniel Weibel
Since the development of BINAP by Noyori, extensive research has been accomplished using this chiral ligand. BINAP proved to be one of the most versatile ligands, catalyzing a wide range of reactions, from hydrogenations to Heck cyclizations.1
The research team at Takasago International Corporation developed a series of ligands and complexes for a plethora of catalyzed asymmetric reactions. Based on a biphenyl architecture, several phosphine ligands have been synthesized. Two large families of ligands can be distinguished, BINAP and SEGPHOS (Figure 1). BINAP is based on a bisnaphthalene backbone with different phosphine derivatives. SEGPHOS is based on a bis(1,3‑benzodioxole) backbone with different phosphine substituents. BINAP and SEGPHOS are highly reactive and selective in a variety of asymmetric hydrogenations. In conjunction with ruthenium, rhodium, palladium and copper complexes, these ligands allow for enantioselectivities of up to >99%.

Figure 1.
Rhodium-Catalyzed 1,4‑Addition of Arylboronic Acids to Coumarins
Chen et al. presented the asymmetric synthesis of arylated coumarins.2 The importance of this transformation with coumarin precursors is illustrated with the synthesis of (R)-tolterodine, a urological drug that can be readily obtained following the asymmetric arylation. Using SEGPHOS and Rh(acac)(C2H4)2, the researchers were able to obtain the desired arylated coumarins with a yield of 88% and ee of 99.6% (Scheme 1).
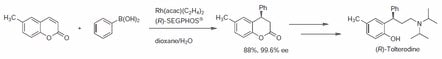
Scheme 1.
Copper-Catalyzed Asymmetric Alkenylation and Phenylation
Tomita et al. developed a new catalytic enantioselective method for chiral alcohol and diarylmethanol synthesis.3 This new method is a great alternative to the kinetic resolution using the Sharpless epoxidation or the asymmetric addition of alkenylzinc reagents to carbonyl compounds, for the synthesis of chiral allylic alcohols. Furthermore, the transformation can be achieved using air- and moisture-stable alkenylsilanes. Using CuF2. H2O with DTBM-SEGPHOS, the aryl aldehyde is reacted with a variety of trimethoxysilane derivatives (Scheme 2). Excellent enantioselectivities were obtained from a wide range of aldehydes.

Scheme 2.
Copper-Catalyzed Asymmetric Hydrosilylations
Lipshutz et al. developed a new method for asymmetric hydrosilylations of various ketones using a preformed DTBM-SEGPHOS. CuH species in the presence of excess polymethylhydrosiloxane.4 High yields and ee's were reported for a variety of ketones (Scheme 3). These chiral building blocks are precursors for the synthesis of various drugs and naturally occurring molecules.

Scheme 3.
Ruthenium-Catalyzed Asymmetric Hydrogenation of Amino Ketones
Ohkuma et al. reported a practical asymmetric hydrogenation reaction using mild hydrogen pressure with a wide scope.5 The access to chiral alcohols from amino ketones is important for the synthesis of physiologically active compounds. In this new process, a diphosphine/ diamine Ru catalyst is utilized. This complex is based on DM-BINAP and 1,1‑di-4‑anisyl-2‑isopropyl-1,2‑ethylenediamine (DAIPEN) and a ruthenium complex. Using a loading as low as 0.01 mol% of catalyst, the hydrogenation of various amino ketones was conducted with up to 99% yield and up to 99.8% ee (Scheme 4). To illustrate the relevance of this new catalyst, Okuma et al. synthesized a series of known molecules used in drug synthesis.

Scheme 4.
Ruthenium-Catalyzed Asymmetric Reductive Amination
Shimizu and coworkers reported a new protocol en route to chiral amino acid derivatives via reductive amination.6 Using a Ru-DM-SEGPHOS complex, a variety of amino esters were obtained in one step from the corresponding ketoesters in highly enantioselective fashion (up to >99% ee) (Scheme 5).

Scheme 5.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?