85325
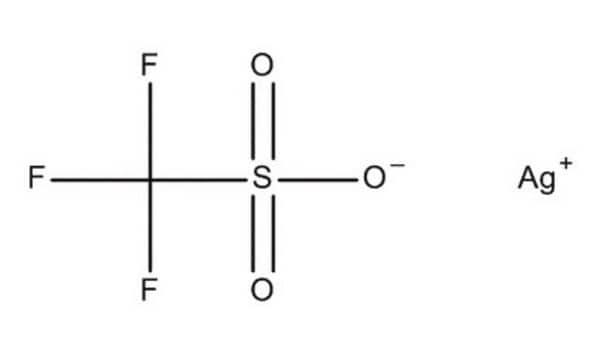
Silver trifluoromethanesulfonate
purum, ≥98.0% (Ag)
Synonym(s):
Ag(OTf), Silver (trifluoromethyl)sulfonate, Silver triflate, Trifluoromethanesulfonic acid silver salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3SO3Ag
CAS Number:
Molecular Weight:
256.94
Beilstein:
3598402
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (Ag)
form
crystals
reaction suitability
core: silver
reagent type: catalyst
SMILES string
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
QRUBYZBWAOOHSV-UHFFFAOYSA-M
Related Categories
General description
Silver trifluoromethanesulfonate p-complexes of monoenes, dienes, trienes, monoynes and diynes have been prepared. It reacts with 2-fluoro- and 3-fluoro-4-alkoxystilbazoles to afford the mesomorphic complexes. Iodine monochloride/AgOTf constitutes an efficient promoter system for the O-glycoside synthesis.
Application
Silver trifluoromethanesulfonate (AgOTf) may be employed as a reagent during glucosylation of several alcohols. AgOTf in combination with p-nitrobenzenesulfenyl chloride may be employed as an activator for the glycosylation.
It may be used for the synthesis of the following:
It may be used for the synthesis of the following:
- cystine-containing peptides
- 3-aminoalkylated indoles
- benzo[b]oxepines and 2H-chromenes
- diversely substituted iminoimidazoazines
Other Notes
Reagent for the substitution of halides by triflate.; Reagent for the glycosylation of glycosyl halides; Reagent used for the deprotection of protected thiols
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Disulfide bond formation in S-acetamidomethyl cysteine-containing peptides by the combination of silver trifluoromethanesulfonate and dimethylsulfoxide/aqueous HCl.
Tamamura H, et al.
Tetrahedron Letters, 34(31), 4931-4934 (1993)
The glucosylation of several alcohols with tetra-O-benzyl-. ALPHA.-D-glucopyranose and a mixture of p-nitrobenzenesulfonyl chloride, silver trifluoromethanesulfonate, and triethylamine.
Koto S, et al.
Bulletin of the Chemical Society of Japan, 53(6), 1761-1762 (1980)
P.J. Garegg et al.
Acta Chemica Scandinavica. Series B, 33, 116-116 (1979)
V. Pozsgay et al.
The Journal of Organic Chemistry, 46, 3761-3761 (1981)
David Crich et al.
Carbohydrate research, 343(10-11), 1858-1862 (2008-04-01)
p-Nitrobenzenesulfenyl chloride is a stable commercially available sulfenyl chloride that, in conjunction with silver triflate, cleanly activates a wide range of thioglycosides for glycosylation at -78 degrees C in CH(2)Cl(2).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service