483346
Silver trifluoromethanesulfonate
≥99.95% trace metals basis
Synonym(s):
AgOTf, Silver triflate, Trifluoromethanesulfonic acid silver salt
About This Item
Recommended Products
Assay
≥99.95% trace metals basis
reaction suitability
core: silver
reagent type: catalyst
mp
286 °C (lit.)
SMILES string
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
QRUBYZBWAOOHSV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Silver trifluoromethanesulfonate (AgOTf ) is a reactive triflating agent, which converts alkyl, acyl and sulfonyl halides to corresponding triflate species.
- It is a highly suitable electrophile to initiate acetylenic oxy-Cope rearrangement of substituted 5-hexen-1-yn-3-ols to synthesize corresponding α,δ-diethylenic aldehydes.
- It can also be used in the diastereoselective cyclization of amino ketenes where the diastereoselectivity depends on Ag(I) concentration.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








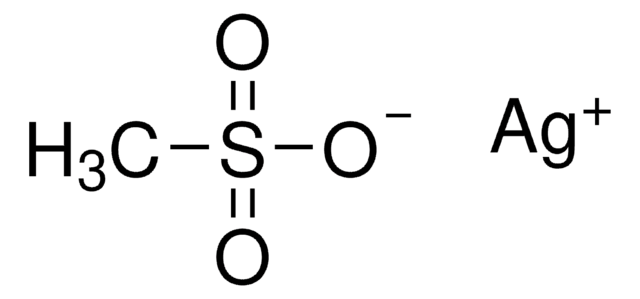


![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)

