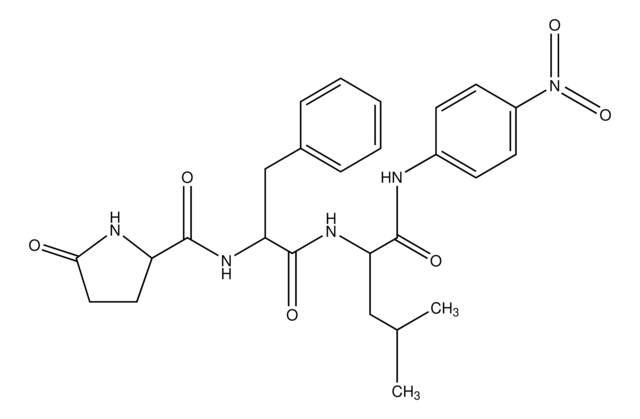
SCP0108
Cathepsin B Substrate
≥95% (HPLC), lyophilized
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C26H36N10O6
Poids moléculaire :
584.63
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.32
Produits recommandés
product name
Cathepsin B Substrate, colorimetric,
Pureté
≥95% (HPLC)
Forme
lyophilized
Composition
Peptide Content, ≥80%
Conditions de stockage
protect from light
Température de stockage
−20°C
Amino Acid Sequence
Z-Arg-Arg-pNA
Description générale
Cathepsin B, a bilobal protein, belongs to the papain-like family of cysteine proteases. It is produced as a preproenzyme. Its catalytic site is present at the interface between the two lobes. Cathepsin B has an occluding loop. It is located in the secretory vesicles of the neuronal cells. Active cathepsin B is found in the endosomal or lysosomal compartment under normal physiological conditions.
Actions biochimiques/physiologiques
Cathepsin B is a lysosomal cysteine proteinase that metabolizes important molecules such as β-amyloid precursor protein into harmless fragments. Cathepsin B may be detected using the chromogenic substrate Z-Arg-Arg-pNA (z-arg-arg-p-nitroanalide) or flourogenic substrate Z-Arg-Arg-AMC (z-Arg-Arg-amino-4-methylcoumarin).
Cathepsin B possesses endopeptidase and exopeptidase activity. Active cathepsin B is mostly engaged in intracellular and extracellular protein turnover, which helps cells maintain homeostatic metabolic activity. It also participates in the regulation of pro-hormone and pro-enzyme activation, antigen processing, inflammatory reactions against antigens, tissue remodeling, and apoptosis. Cathepsin B plays a key role in acute pancreatitis. It also plays a vital role in lipid metabolism in atherosclerosis.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
S Hasnain et al.
The Journal of biological chemistry, 268(1), 235-240 (1993-01-05)
The pH dependence of cathepsin B-catalyzed hydrolyzes is very complex. At least seven dissociable groups are involved in the binding and hydrolysis of 7-amido-4-methyl coumarin and p-nitroaniline (pNA)-based substrates containing a P1 Arg and either a Phe or Arg at
Cathepsin B: Basis Sequence: Mouse.
Dora Cavallo-Medved et al.
The AFCS-nature molecule pages, 2011 (2011-01-01)
Yasuhito Sako et al.
Experimental parasitology, 127(3), 693-701 (2010-11-26)
Cysteine peptidases have potent activities in the pathogenesis of various parasitic infections, and are considered as targets for chemotherapy and antigens for vaccine. In this study, two cathepsin B-like cysteine peptidases (EmCBP1 and EmCBP2) from Echinococcus multilocularis metacestodes were identified
Xiaohua Peng et al.
Journal of immunology (Baltimore, Md. : 1950), 209(7), 1314-1322 (2022-09-28)
Postviral bacterial infections are a major health care challenge in coronavirus infections, including COVID-19; however, the coronavirus-specific mechanisms of increased host susceptibility to secondary infections remain unknown. In humans, coronaviruses, including SARS-CoV-2, infect lung immune cells, including alveolar macrophages, a
Angela J Eykelbosh et al.
Comparative biochemistry and physiology. Part A, Molecular & integrative physiology, 156(2), 218-223 (2010-02-23)
This study presents evidence that cathepsin B, a lysosomal protease, may be involved in the regulation of apoptosis during serum-starvation in teleost follicles. Zebrafish vitellogenic follicles were isolated, incubated under serum-free conditions and homogenized. The follicle extracts demonstrated caspase-3-like activity
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique