E5630
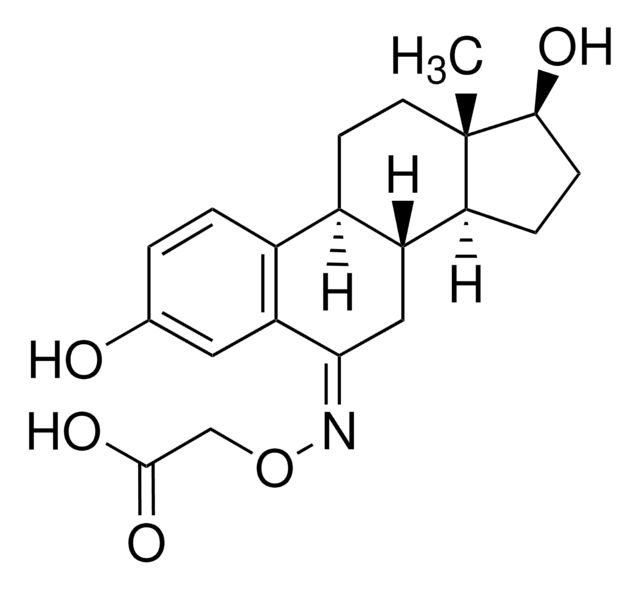
β-Estradiol 6-(O-carboxymethyl)oxime: BSA
About This Item
Produits recommandés
Stérilité
non-sterile
Niveau de qualité
Forme
lyophilized powder
Ampleur du marquage
~30 mol steroid per mol BSA
Solubilité
phosphate buffer: 0.90-1.10 mg/mL, faintly hazy to hazy, colorless to faintly yellow
Conditions d'expédition
ambient
Température de stockage
2-8°C
Description générale
Application
- to test its effect on the N-methyl-D-aspartate receptor (NMDAR) currents in female dorsal root ganglion (DRG) neurons
- to coat microplates for the detection of plasma 17β-Estradiol levels using indirect enzyme-linked immunosorbent assay (ELISA)
- to test its effect on MutL homolog 1 in colorectal cancer lines
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique