19176
DL-Buthionine-sulfoximine
≥99.0% (TLC)
Synonyme(s) :
DL-Buthionine (S,R)-sulfoximine, BSO, Buthionine sulfoximine, Butionine sulfoximine
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Essai
≥99.0% (TLC)
Forme
powder or crystals
Pf
215 °C
Solubilité
H2O: 50 mg/mL, clear to almost clear, colorless
Température de stockage
2-8°C
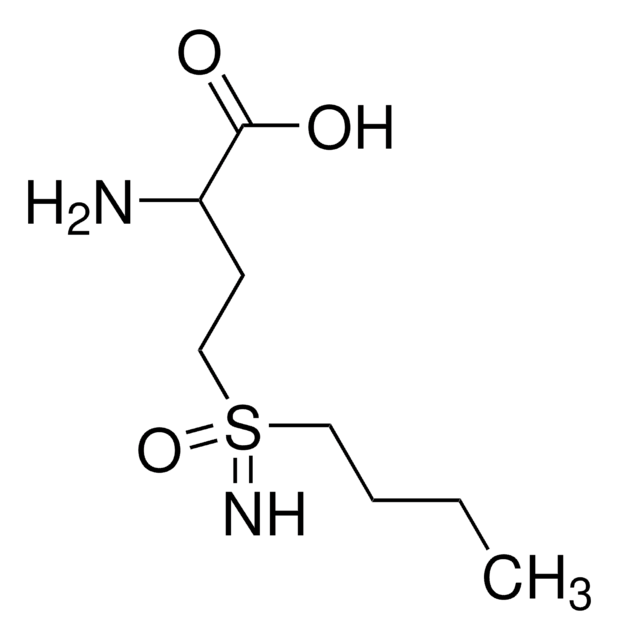
Chaîne SMILES
CCCCS(=N)(=O)CCC(N)C(O)=O
InChI
1S/C8H18N2O3S/c1-2-3-5-14(10,13)6-4-7(9)8(11)12/h7,10H,2-6,9H2,1H3,(H,11,12)
Clé InChI
KJQFBVYMGADDTQ-UHFFFAOYSA-N
Application
- examine whether the inhibition of glutathione by BSO enhances the apoptotic effect of estrogen on antihormone-resistant human breast cancer cells[1]
- investigate the effect of BSO on development of bovine embryos[2]
- inhibit GSH in several studies[3][4]
- investigate the effect of GSH synthesis on oocyte maturation[5]
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique