791056P
Avanti
2-OHOA
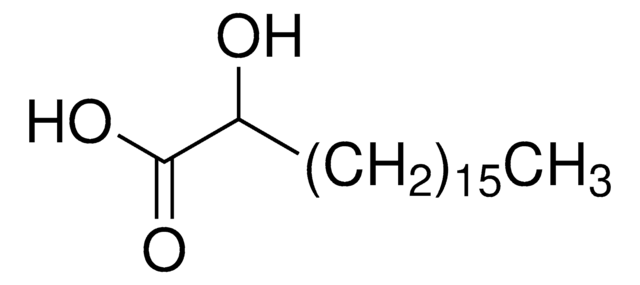
2-hydroxyoleic acid (sodium salt), powder
Synonyme(s) :
Minerval; NaCHOleate; 2OHOA
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C18H33NaO3
Numéro CAS:
Poids moléculaire :
320.44
Code UNSPSC :
12352211
Nomenclature NACRES :
NA.25
Produits recommandés
Pureté
>99% (TLC)
Forme
powder
Conditionnement
pkg of 1 × 1 g (791056P-1g)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand 791056P
Type de lipide
neutral lipids
neutral glycerides
Conditions d'expédition
dry ice
Température de stockage
−20°C
Description générale
2-hydroxyoleic acid (2-OHOA) is a synthetic and non-β oxidation-metabolizable oleic acid derivative. It contains 18 carbon atoms in its fatty acid chain length.
Application
2-hydroxyoleic acid (2-OHOA) is suitable for use:
- to study its antihypertensive action in adult spontaneously hypertensive rats (SHRs)
- to study the chemical structure-effect relation of 2-OHOA in the reduction of body weight in rats
- as an antitumor compound, to study its effect on xenografts lipidome based on imaging mass spectrometry (IMS)
Actions biochimiques/physiologiques
2-Hydroxy Oleic Acid is an inducer of cell cycle arrest and apoptosis in several cancer cell lines, including glioma, leukemia, breast and colon cancer lines. 2-Hydroxy Oleic Acid increases sphingomyelin (SM) levels in the membranes of tumor cells, which typically display decreased SM membrane content, and remodeled membranes, compared with normal cells. The compound has no effect on SM levels in non-cancer cells.
2-hydroxyoleic acid (2-OHOA) acts as a potential antihypertensive agent. Oral administration of 2-OHOA is used to reduce body weight.
Conditionnement
20 mL Clear Glass Screw Cap Vial (791056P-1g)
Informations légales
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Code de la classe de stockage
11 - Combustible Solids
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Structure-effect relation of C18 long-chain fatty acids in the reduction of body weight in rats
Vogler O, et al.
International Journal of Obesity, 32(3), 464-464 (2008)
Alena Khmelinskaia et al.
Langmuir : the ACS journal of surfaces and colloids, 30(8), 2117-2128 (2014-02-05)
Recent research regarding 2-hydroxylated fatty acids (2OHFAs) showed clear evidence of their benefits in the treatment of cancer, inflammation, and neurodegenerative disorders such as Alzheimer's disease. Monolayer compressibility isotherms and isothermal titration calorimetry of 2OHFA (C18-C22) in phosphatidylcholine/phosphatidylethanolamine/sphingomyelin/cholesterol (1:1:1:1 mole
Silvia Terés et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(22), 8489-8494 (2012-05-16)
Despite recent advances in the development of new cancer therapies, the treatment options for glioma remain limited, and the survival rate of patients has changed little over the past three decades. Here, we show that 2-hydroxyoleic acid (2OHOA) induces differentiation
Victoria Lladó et al.
Biochimica et biophysica acta, 1838(6), 1619-1627 (2014-02-15)
This review summarizes the cellular bases of the effects of NaCHOleate (2-hydroxyoleic acid; 2OHOA; Minerval) against glioma and other types of tumors. NaCHOleate, activates sphingomyelin synthase (SGMS) increasing the levels of cell membrane sphingomyelin (SM) and diacylglycerol (DAG) together with
Victoria Llado et al.
Journal of cellular and molecular medicine, 14(3), 659-670 (2009-05-06)
Minerval is an oleic acid synthetic analogue that impairs lung cancer (A549) cell proliferation upon modulation of the plasma membrane lipid structure and subsequent regulation of protein kinase C localization and activity. However, this mechanism does not fully explain the
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique