E4402
2-Ethoxybenzamide
97%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
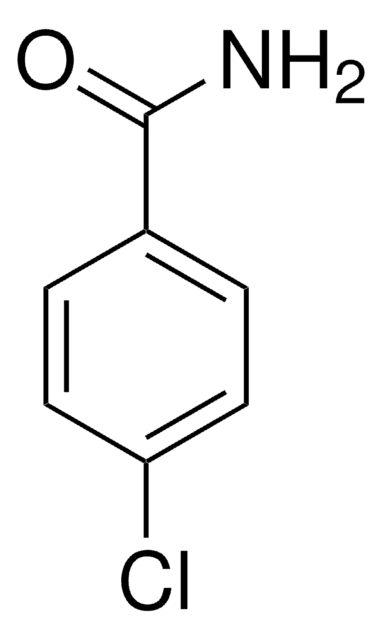
Formule linéaire :
C2H5OC6H4CONH2
Numéro CAS:
Poids moléculaire :
165.19
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
97%
Pf
132-134 °C (lit.)
Chaîne SMILES
CCOc1ccccc1C(N)=O
InChI
1S/C9H11NO2/c1-2-12-8-6-4-3-5-7(8)9(10)11/h3-6H,2H2,1H3,(H2,10,11)
Clé InChI
SBNKFTQSBPKMBZ-UHFFFAOYSA-N
Catégories apparentées
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Yoshihiro Hayashi et al.
Pharmaceutics, 12(7) (2020-07-02)
We previously reported a novel method for the precise prediction of tablet properties (e.g., tensile strength (TS)) using a small number of experimental data. The key technique of this method is to compensate for the lack of experimental data by
N Hirasawa et al.
Chemical & pharmaceutical bulletin, 47(3), 417-420 (1999-04-23)
Solid dispersions of carbamazepine or ethenzamide were prepared by melting and rapid cooling with liquid nitrogen using lactose as a carrier. The physical characteristics of these solid dispersions were investigated by powder X-ray diffraction, differential scanning calorimetry, and dissolution rate
Y Miyamoto et al.
Chemical & pharmaceutical bulletin, 46(9), 1432-1437 (1998-10-17)
A computer optimization technique based on surface response methodology was applied to optimize the wet granulation process for designing tablets. Physical properties (mean granule size, granule size distribution, compressibility, granule strength) of a model granule formulation containing ethenzamide were accurately
Tadashi Fukunaka et al.
Journal of pharmaceutical sciences, 94(5), 1004-1012 (2005-03-29)
Milling is a common procedure to improve bioavailability of many active pharmaceutical ingredients (APIs), which typically have low solubility in water. But such micronization can yield an increase in the cohesiveness of particles. Although particle cohesiveness is desirable for tablet
H Uehara et al.
Cancer letters, 135(1), 83-90 (1999-03-17)
Six-week-old male F344 rats were given a mixture of 0.01% diethylnitrosamine, 0.05% N-butyl-N-(4-hydroxybutyl)nitrosamine and 0.02% N-methyl-N'-nitro-N-nitrosoguanidine in their drinking water for 1 week. When 0.8%, 0.4%, or 0% of a mixture of non-steroidal anti-inflammatory drugs (NSAIDs) (acetaminophen, aspirin, dipyrone plus
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique