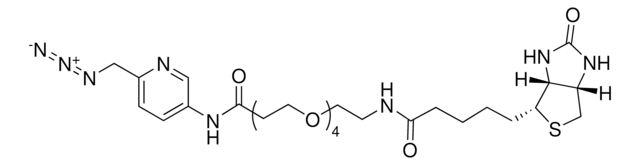
924229
(S,R,S)-AHPC-Me
95%
Synonyme(s) :
(2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-((S)-1-(4-(4-methylthiazol-5-yl)phenyl)ethyl)pyrrolidine-2-carboxamide, PROTAC® research ligand, VH032 methyl derivative
About This Item
Produits recommandés
ligand
VH032
Niveau de qualité
Pureté
95%
Forme
powder
Température de stockage
2-8°C
Chaîne SMILES
C([C@H](C(C)(C)C)N)(=O)N1[C@H](C(N[C@@H](C)C2=CC=C(C=C2)C3=C(C)N=CS3)=O)C[C@@H](O)C1
Application
Related Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Browse our growing synthesis and research tools: Protein Degrader Building Blocks
Autres remarques
Discovery of ARD-69 as a highly potent proteolysis targeting chimera (PROTAC) degrader of androgen receptor (AR) for the treatment of prostate cancer
Design, Synthesis, and Biological Evaluation of MEK PROTACs
Antibody–PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4
Discovery of a First-in-Class Mitogen-Activated Protein Kinase Kinase 1/2 Degrader
Discovery of PROTAC BCL-XL degraders as potent anticancer agents with low on-target platelet toxicity
Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression
A caged E3 ligase ligand for PROTAC-mediated protein degradation with light
Discovery of SHP2-D26 as a First, Potent, and Effective PROTAC Degrader of SHP2 Protein
Selective CDK6 degradation mediated by cereblon, VHL, and novel IAP-recruiting PROTACs
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique