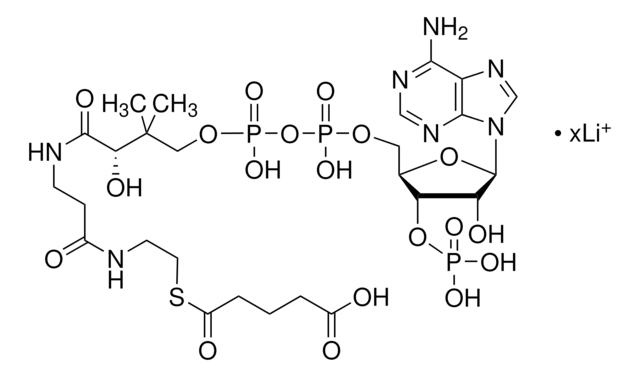
As reported in the PROPERTIES section, this product is soluble in H2O at 50 mg/mL yielding a clear, colorless solution.
M4263
Malonyl coenzyme A lithium salt
≥90% (HPLC)
Synonyme(s) :
Malonyl CoA lithium salt
Sélectionner une taille de conditionnement
180,00 $
Sélectionner une taille de conditionnement
About This Item
180,00 $
Produits recommandés
Essai
≥90% (HPLC)
Forme
powder
Solubilité
H2O: soluble 50 mg/mL protein, clear, colorless
Température de stockage
−20°C
Chaîne SMILES
[Li].CC(C)(COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP(O)(O)=O)n2cnc3c(N)ncnc23)C(O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O
InChI
1S/C24H38N7O19P3S.Li/c1-24(2,19(37)22(38)27-4-3-13(32)26-5-6-54-15(35)7-14(33)34)9-47-53(44,45)50-52(42,43)46-8-12-18(49-51(39,40)41)17(36)23(48-12)31-11-30-16-20(25)28-10-29-21(16)31;/h10-12,17-19,23,36-37H,3-9H2,1-2H3,(H,26,32)(H,27,38)(H,33,34)(H,42,43)(H,44,45)(H2,25,28,29)(H2,39,40,41);
Clé InChI
OPIJLICRFQMMJH-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- in Krebs Ringer bicarbonate medium for preincubation of trypsinized and re-suspended fibroblast for fatty acid oxidation assay[1]
- in HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer used for scintillation proximity assay for fatty acid synthase[2]
- as an internal standard in the reaction mixture used for succinyl-CoA ligase assay[3]
Actions biochimiques/physiologiques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
-
whats the appropriate solvent? PBS? DMSO? DW?
1 answer-
Helpful?
-
-
Malonyl Coenzyme A can be store as aqueous solution ?
1 answer-
The stability of Malonyl Coenzyme A in aqueous solution has not been determined. However, other sources indicate that prepared solutions may be stable for several months at a minimum of -20 °C.
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique