A5964
Monoclonal Anti-Phosphotyrosine–Peroxidase antibody produced in mouse
clone PT-66, purified immunoglobulin, lyophilized powder
Synonyme(s) :
Monoclonal Anti-Phosphotyrosine, Phospho-Tyr, Phospho-tyrosine, p-Tyr
About This Item
Produits recommandés
Source biologique
mouse
Niveau de qualité
Conjugué
peroxidase conjugate
Forme d'anticorps
purified immunoglobulin
Type de produit anticorps
primary antibodies
Clone
PT-66, monoclonal
Forme
lyophilized powder
Conditionnement
vial of 0.2 mL conjugate
Technique(s)
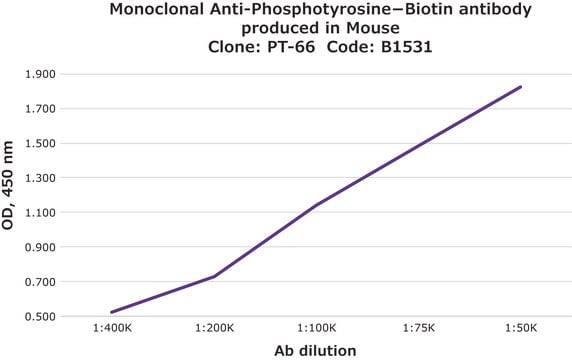
direct ELISA: 1:60,000 using Phosphotyrosine-BSA
dot blot: 1:40,000-1:200,000 using phosphotyrosine-BSA using chromogenic and chemiluminescent substrates, respectively
Isotype
IgG1
Température de stockage
2-8°C
Modification post-traductionnelle de la cible
unmodified
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Spécificité
Immunogène
Application
- enzyme linked immunosorbent assay (ELISA)
- dot blot
- Chemiluminescence dot blot
- kinase assay
Actions biochimiques/physiologiques
Forme physique
Clause de non-responsabilité
Not finding the right product?
Try our Outil de sélection de produits.
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique