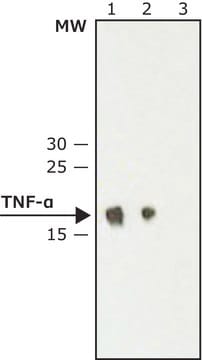
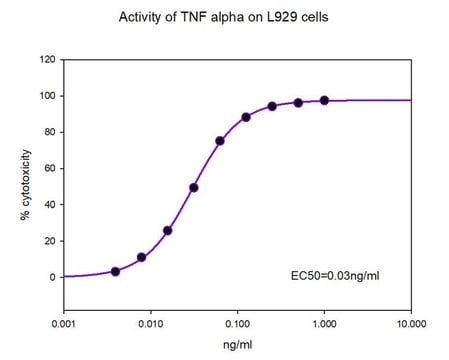
Recommendations against the long-term storage and use of this standard are listed in the associated Information Leaflet published by the European Pharmacopoeia. From the leaflet:
"The container should not be opened until required for use. Allow the closed container to equilibrate at ambient temperature before opening to avoid uptake of moisture. This substance has been freeze-dried and is known to be hygroscopic. Tap the container gently to collect the material at the bottom. Reconstitute the contents by injecting a suitable volume of the solvent. Mix gently to ensure complete dissolution. When necessary transfer the solution to an appropriate volumetric flask and dilute to the required concentration. Ph. Eur. RS are for immediate use. Once the container has been opened, its entire content must be used immediately. Any further storage and re-use are not warranted."