80614
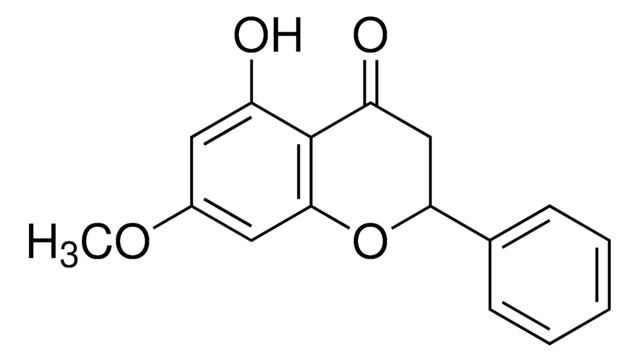
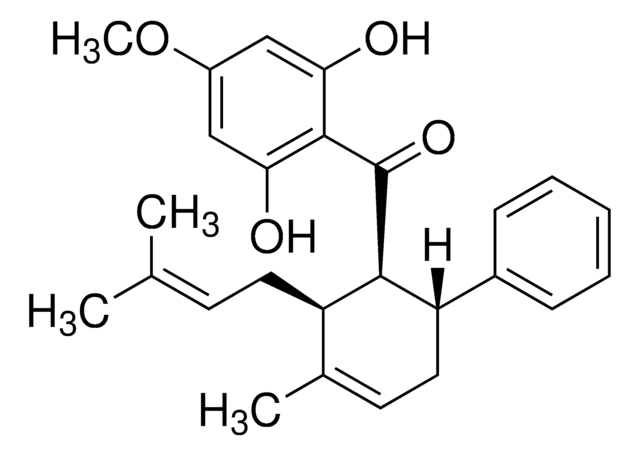
Pinostrobin
≥99.0% (TLC)
Synonyme(s) :
(S)-2,3-Dihydro-5-hydroxy-7-methoxy-2-phenyl-4H-1-benzopyran-4-one
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C16H14O4
Numéro CAS:
Poids moléculaire :
270.28
Numéro Beilstein :
270230
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352200
ID de substance PubChem :
Produits recommandés
Pureté
≥99.0% (TLC)
Chaîne SMILES
COc1cc(O)c2C(=O)C[C@H](Oc2c1)c3ccccc3
InChI
1S/C16H14O4/c1-19-11-7-12(17)16-13(18)9-14(20-15(16)8-11)10-5-3-2-4-6-10/h2-8,14,17H,9H2,1H3/t14-/m0/s1
Clé InChI
ORJDDOBAOGKRJV-AWEZNQCLSA-N
Actions biochimiques/physiologiques
Elicits intense apoptotic response from cultured leukemia cells in vitro. Strongly inhibits the Ca2+ signals involved in the control of G2/M phase cell cycle progression in Saccharomyces cerevisiae. Shows potent antiviral effect against herpes simplex virus-1.
Conditionnement
Bottomless glass bottle. Contents are inside inserted fused cone.
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Hadi Poerwono et al.
Bioorganic & medicinal chemistry letters, 20(7), 2086-2089 (2010-03-12)
Pinostrobin (5-hydroxy-7-methoxyflavanone) obtained in relatively large amounts from fingerroot (Boesenbergia pandurata) was converted to its C-6 and C-8 prenylated derivatives. The Mitsunobu reaction, europium(III)-catalyzed Claisen-Cope rearrangement, and Claisen reaction coupled with cross-metathesis were used as the key steps. Using a
Nwet Nwet Win et al.
Journal of natural products, 70(10), 1582-1587 (2007-09-28)
The chloroform extract of rhizomes of Boesenbergia pandurata demonstrated marked preferential cytotoxicity against human pancreatic PANC-1 cancer cells in nutrient-deprived medium. Bioactivity-directed investigation of this extract yielded four new secondary metabolites, geranyl-2,4-dihydroxy-6-phenethylbenzoate ( 1), 2',4'-dihydroxy-3'-(1''-geranyl)-6'-methoxychalcone ( 2), (1' R,2' S,6'
J C Le Bail et al.
Cancer letters, 156(1), 37-44 (2000-06-07)
The interaction between the estrogen receptor and 5-hydroxy-7-methoxyflavanone (pinostrobin) was studied in the presence or absence of estradiol or dehydroepiandrosterone sulfate (DHEAS), respectively, using a stably transfected human breast cancer cell line (MVLN). We also evaluated its action on the
Tan Siew Kiat et al.
Bioorganic & medicinal chemistry letters, 16(12), 3337-3340 (2006-04-20)
Boesenbergia rotunda (L.) cyclohexenyl chalcone derivatives, 4-hydroxypanduratin A and panduratin A, showed good competitive inhibitory activities towards dengue 2 virus NS3 protease with the Ki values of 21 and 25 microM, respectively, whilst those of pinostrobin and cardamonin were observed
Chavi Yenjai et al.
Bioorganic & medicinal chemistry letters, 20(9), 2821-2823 (2010-04-07)
Flavones 1-4 isolated from Kaempferia parviflora were used for structural modification. Sixteen flavonoid derivatives, including four new derivatives, were synthesized and evaluated for cytotoxicity against KB and NCI-H187 cell lines. Flavanones 2a-4a demonstrated higher cytotoxic activity than the parent compounds.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique