M-065
(±)-MDEA solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonyme(s) :
(±)-3,4-Methylenedioxyethylamphetamine
About This Item
Produits recommandés
Qualité
certified reference material
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
drug control
Narcotic Licence Schedule D (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
Concentration
1.0 mg/mL in methanol
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
forensics and toxicology
Format
single component solution
Température de stockage
2-8°C
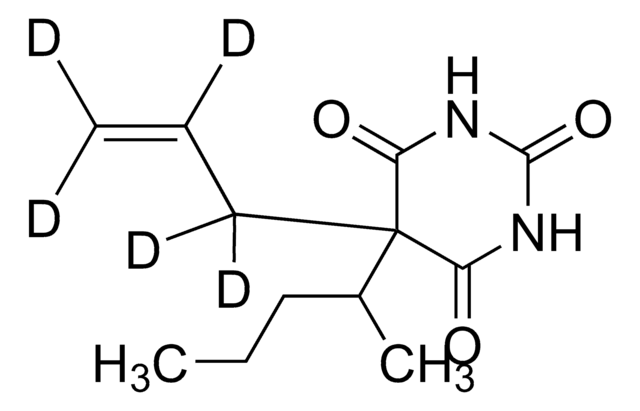
Chaîne SMILES
CC(CC1=CC=C(OCO2)C2=C1)NCC
InChI
1S/C12H17NO2/c1-3-13-9(2)6-10-4-5-11-12(7-10)15-8-14-11/h4-5,7,9,13H,3,6,8H2,1-2H3
Clé InChI
PVXVWWANJIWJOO-UHFFFAOYSA-N
Description générale
Application
- (±)-MDEA Solution for Neurotransmitter Research: Utilized extensively in the study of serotonin release mechanisms, (±)-MDEA-D3 provides a robust tool for examining the biochemical pathways involved in neurotransmitter dynamics, essential for advancements in neuropharmacology (Wen et al., 2024).
- Racemic MDEA Reagent for Pharmacological Studies: This solution is crucial for the synthesis and pharmacological evaluation of new psychoactive substances, enabling researchers to delineate the metabolic and pharmacodynamic profiles of novel therapeutic agents (Boucenna et al., 2023).
- High-Purity (±)-MDEA-D3 Reference Material: As a reference standard, this high-purity material is vital for calibrating analytical instruments like LC-MS/MS, ensuring accuracy and reproducibility in quantitative drug analysis and forensic toxicology (Ghorbani et al., 2020).
- (±)-MDEA-D3 Internal Standard for LC-MS/MS: This deuterated version of MDEA serves as an internal standard in chromatographic analyses, enhancing the precision of quantitative measurements in complex biological matrices (Tian et al., 2021).
- (±)-MDEA-D3 Solution for Pharmaceutical Cannabinoid Profiling: Employed in cannabinoid biosynthesis research, (±)-MDEA-D3 aids in the profiling and characterization of cannabinoids, supporting the development of cannabis-based pharmaceuticals with targeted therapeutic effects (Irani et al., 2018).
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
49.5 °F - closed cup
Point d'éclair (°C)
9.7 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique