700051P
Avanti
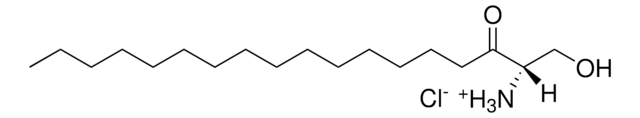
22(S)-hydroxycholesterol-d7
Avanti Research™ - A Croda Brand
Synonyme(s) :
25,26,26,26,27,27,27-heptadeuterocholest-5-ene-3β,22S-diol
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C27H39O2D7
Numéro CAS:
Poids moléculaire :
409.70
Code UNSPSC :
41141804
Nomenclature NACRES :
NA.25
Produits recommandés
Description
cholest-5-ene-3β,22(S)-diol-d7
Pureté
>99% (TLC)
Forme
powder
Conditionnement
pkg of 1 × 1 mg (700051P-1mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand
Conditions d'expédition
dry ice
Température de stockage
−20°C
Description générale
22(S)-hydroxycholesterol is an enantiomer of 22(R)-hydroxycholesterol. 22(S)-hydroxycholesterol-d7 is a deuterated form of 22(S)-hydroxycholesterol.
Application
22(S)-hydroxycholesterol-d7 may be used as an internal standard in liquid chromatography with tandem mass spectrometry (LC-MS-MS) analysis of plasma low-density lipoprotein (LDL).
Actions biochimiques/physiologiques
22(S)-hydroxycholesterol (22(S)-HC) promotes glucose catabolism and uptake and is regarded as a potential target to treat type 2 diabetes. 22(S)-HC also prevents the accumulation of lipids and lipid synthesis in hepatocytes and myotubes. Unlike 22(R)-hydroxycholesterol, 22(S)-HC is not estrogenic and is not a ligand for liver X receptor (LXR).
Conditionnement
5 mL Amber Glass Screw Cap Vial (700051P-1mg)
Informations légales
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Code de la classe de stockage
11 - Combustible Solids
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Hiroyoshi Sato et al.
Bioscience, biotechnology, and biochemistry, 68(8), 1790-1793 (2004-08-24)
In order to test the estrogenic activity of sterol oxidation products from cholesterol and phytosterols, an estrogen-dependent gene expression assay was performed in estrogen receptor alpha-stably transformed HeLa cells. The ranking of the estrogenic potency of these compounds was different:
Myung-Jin Oh et al.
Journal of lipid research, 57(5), 791-808 (2016-03-19)
Endothelial biomechanics is emerging as a key factor in endothelial function. Here, we address the mechanisms of endothelial stiffening induced by oxidized LDL (oxLDL) and investigate the role of oxLDL in lumen formation. We show that oxLDL-induced endothelial stiffening is
Nina Pettersen Hessvik et al.
The Journal of steroid biochemistry and molecular biology, 128(3-5), 154-164 (2011-11-05)
The aim of this study was to explore the effects of 22(S)-hydroxycholesterol (22(S)-HC) on lipid and glucose metabolism in human-derived cells from metabolic active tissues. Docking of T0901317 and 22(S)-HC showed that both substances fitted into the ligand binding domain
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique