B27005
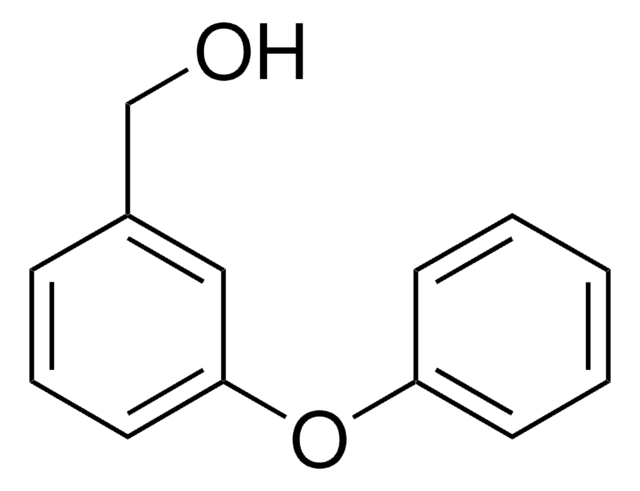
3-Benzyloxybenzaldehyde
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
C6H5CH2OC6H4CHO
Numéro CAS:
Poids moléculaire :
212.24
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
98%
Forme
chunks
Pf
56-58 °C (lit.)
Chaîne SMILES
O=Cc1cccc(OCc2ccccc2)c1
InChI
1S/C14H12O2/c15-10-13-7-4-8-14(9-13)16-11-12-5-2-1-3-6-12/h1-10H,11H2
Clé InChI
JAICGBJIBWDEIZ-UHFFFAOYSA-N
Application
Some of the reported applications of 3-benzyloxybenzaldehyde are:
- Synthesis of silybin analogs as anticancer agents that produce apoptosis in ovarian cancer cells through tubulin inhibition.
- Synthesis of porphyrin and boron dipyrromethene (BODIPY) derivatives for fluorescent applications.
- Preparation of styrene copolymers with varying chain mobilities.
- Synthesis of aromatic derivatives of trimethoprim as potential antimalarial agents.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Synthesis of a novel BODIPY library and its application in the discovery of a fructose sensor.
Zhai D, et al.
ACS Combinatorial Science, 14(2), 81-84 (2012)
Target guided synthesis of 5-benzyl-2, 4-diamonopyrimidines: their antimalarial activities and binding affinities to wild type and mutant dihydrofolate reductases from Plasmodium falciparum.
Sirichaiwat C, et al.
Journal of Medicinal Chemistry, 47(2), 345-354 (2004)
Design and discovery of silybin analogues as antiproliferative compounds using a ring disjunctive?Based, natural product lead optimization approach.
Manivannan E, et al.
European Journal of Medicinal Chemistry, 133, 365-378 (2017)
Nasimossadat Banarouei et al.
Mini reviews in medicinal chemistry, 19(8), 679-687 (2018-04-26)
N-aryl derivatives of phthalimide and 4-nitro phthalimide have demonstrated cyclooxygenase inhibitory activity. Also, they possess excellent analgesic and antiinflammatory activity. In this work, a new series of N-arylmethylideneamino derivatives of phthalimide and 4-nitro phthalimide were designed and synthesized. The designed
Synthesis of Functionalized trans?A2B2?Porphyrins Using Donor?Acceptor Cyclopropane?Derived Dipyrromethanes.
Beyzavi M H, et al.
Advanced Synthesis & Catalysis, 355(7), 1409-1422 (2013)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique