916714
A1V2PF2-NHEt
≥95%
Synonyme(s) :
(S)-1-((S)-2-((S)-2-Aminopropanamido)-2-cyclohexylacetyl)-N-((S)-1-(ethylamino)-3-(4-fluorophenyl)-1-oxopropan-2-yl)pyrrolidine-2-carboxamide, AVP ligand, IAP E3 ligase lead for protein degrader research, SNIPER building block
Sélectionner une taille de conditionnement
735,00 $
Sélectionner une taille de conditionnement
About This Item
735,00 $
Produits recommandés
ligand
A1V2PF2
Niveau de qualité
Essai
≥95%
Forme
powder
Pertinence de la réaction
reagent type: ligand
Groupe fonctionnel
amine
Température de stockage
2-8°C
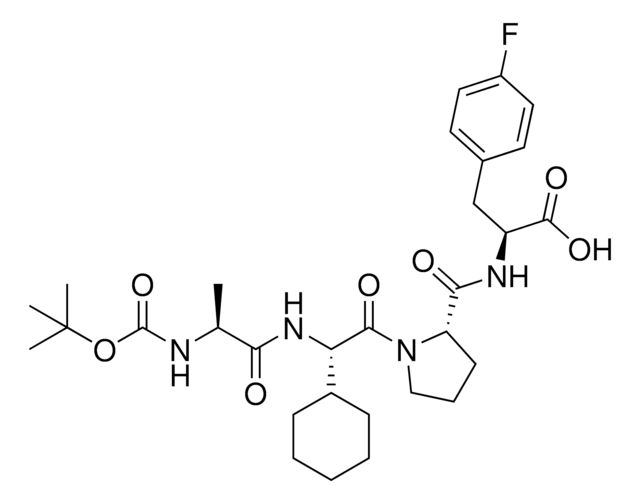
Chaîne SMILES
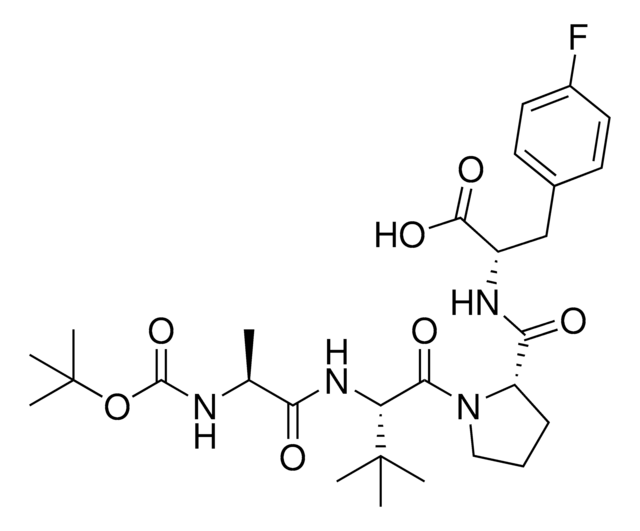
N[C@H](C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(NCC)=O)CC2=CC=C(C=C2)F)=O)=O)C3CCCCC3)=O)C
InChI
1S/C27H40FN5O4/c1-3-30-25(35)21(16-18-11-13-20(28)14-12-18)31-26(36)22-10-7-15-33(22)27(37)23(32-24(34)17(2)29)19-8-5-4-6-9-19/h11-14,17,19,21-23H,3-10,15-16,29H2,1-2H3,(H,30,35)(H,31,36)(H,32,34)/t17-,21-,22-,23-/m0/s1
Clé InChI
FHMJMSLAQVUJGX-ZMVGRULKSA-N
Catégories apparentées
Application
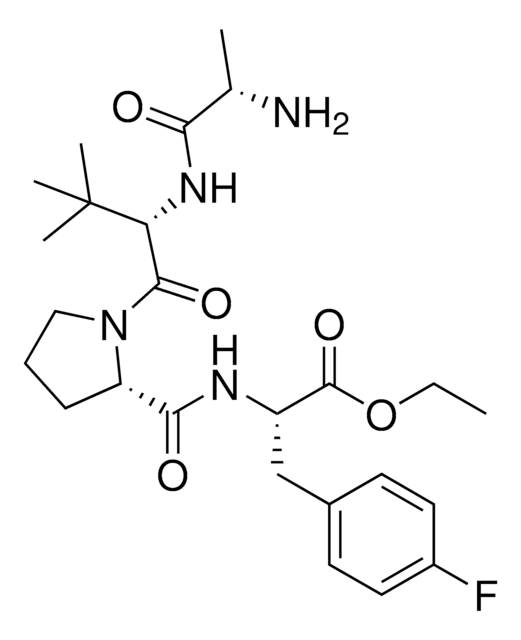
A1V2PF2-NHEt conjugates are also available for degrader synthesis. Browse our full synthesis offering here for streamlining SNIPER and PROTAC® degrader libraries: Degrader Building Blocks with Inhibitor of Apoptosis Protein (IAP) In Silico-Derived Ligands
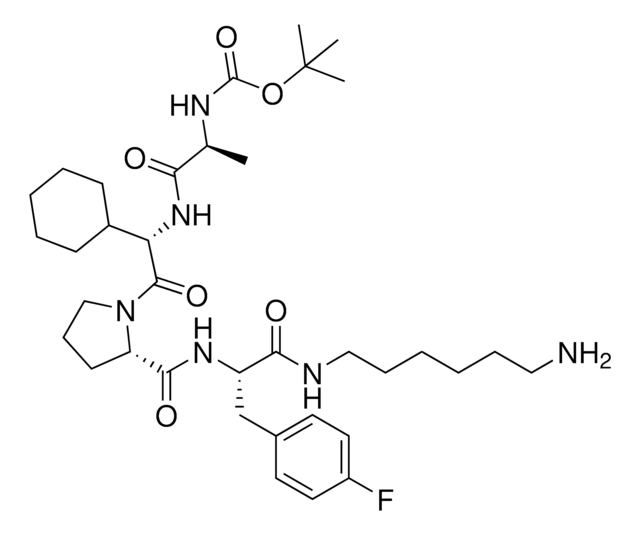
917931 A1V2PF2-NHEt-C6-NH2
916684 A1V2PF2-NHEt-C10-NH2
916935 A1V2PF2-NHEt-PEG1-NH2
917192 A1V2PF2-NHEt-PEG3-NH2
Autres remarques
In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs)
SNIPERs−Hijacking IAP activity to induce protein degradation
E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Targeted protein degradation reduces disease-relevant proteins in cells using small molecules, hijacking endogenous proteolysis systems.
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique