900624
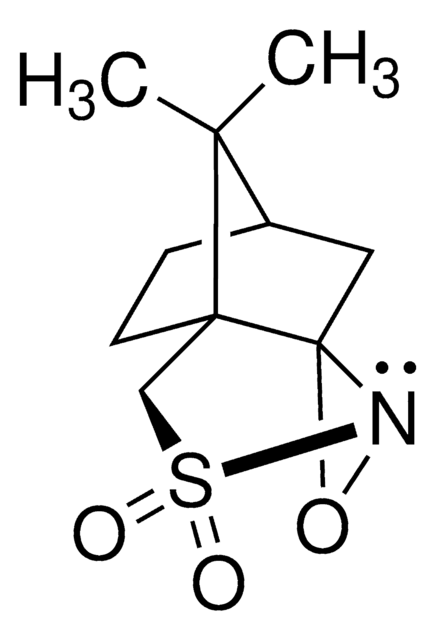
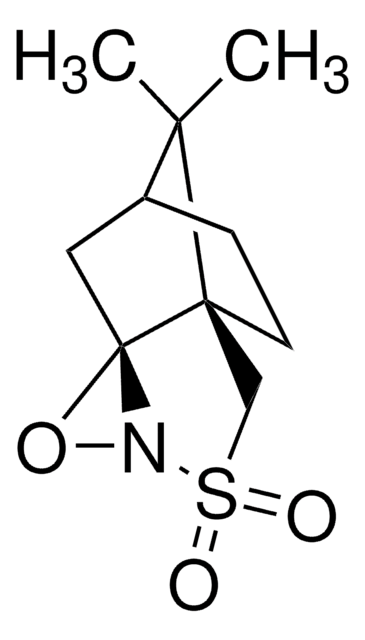
Di-t-butyl oxaziridine
≥95%
Synonyme(s) :
Kurti oxaziridine
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥95%
Forme
liquid
Disponibilité
available only in USA
Indice de réfraction
n/D 1.4453
Densité
0.90 g/mL
Température de stockage
2-8°C
Chaîne SMILES
CC(C)(C)C1(NO1)C(C)(C)C
Application
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Self-react. C
Code de la classe de stockage
5.2 - Organic peroxides and self-reacting hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
143.6 °F
Point d'éclair (°C)
62 °C
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Contenu apparenté
Amines and their derivatives are ubiquitous substances since they make up the overwhelming majority of drug molecules, agrochemicals as well as many compounds that are produced by plants and living organisms (i.e., natural products).
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique