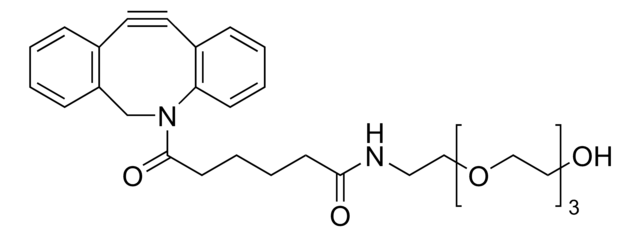
761524
Dibenzocyclooctyne-N-hydroxysuccinimidyl ester
for Copper-free Click Chemistry
Synonyme(s) :
DBCO-NHS ester, DBCO-SE
About This Item
Produits recommandés
Forme
solid
Capacité de réaction
reaction type: click chemistry
Pertinence de la réaction
reagent type: cross-linking reagent
Pf
149-157 (decomposition)
Groupe fonctionnel
NHS ester
Température de stockage
−20°C
Chaîne SMILES
O=C(CCC(ON(C(CC1)=O)C1=O)=O)N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4
InChI
1S/C23H18N2O5/c26-20(13-14-23(29)30-25-21(27)11-12-22(25)28)24-15-18-7-2-1-5-16(18)9-10-17-6-3-4-8-19(17)24/h1-8H,11-15H2
Clé InChI
XCEBOJWFQSQZKR-UHFFFAOYSA-N
Description générale
Application
- In the modification and labeling of biomolecules such as antibodies and streptavidin by introducing DBCO groups onto their surfaces
- As a cross linker to conjugate peptide antigens onto the surface of poly(lactic-co-glycolic acid) (PLGA) nanoparticles
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique



![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)

![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)



![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)