Regioregular means all of the monomer units are in the same orientation. E.g. if you think of each monomer unit as your palm, regioregular would have all palms facing away, fingers up - all in same orientation. (Regiorandom might have one pointing up, one pointing down, one with palms facing you, one with palms facing away, etc, all in a row.)
445703
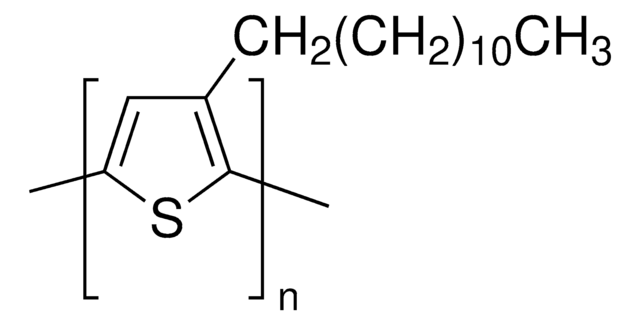
Poly(3-hexylthiophene-2,5-diyl)
regioregular
Synonyme(s) :
P3HT
Sélectionner une taille de conditionnement
867,00 $
Sélectionner une taille de conditionnement
About This Item
867,00 $
Produits recommandés
Niveau de qualité
Poids mol.
average Mw 50,000-100,000
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Conductivité
~103 S/cm (when doped with iodine)
Pf
238 °C
238 °C
Fluorescence
λex 443 nm; λem 568 nm in chloroform
Énergie orbitale
HOMO 5 eV
LUMO 3 eV
Performance des dispositifs OPV
ITO/NiO/P3HT/PC61BM/LiF/Al
- Short-circuit current density (Jsc): 11.3 mA/cm2
- Open-circuit voltage (Voc): 0.64 V
- Fill Factor (FF): 0.69
- Power Conversion Efficiency (PCE): 5.16 %
ITO/PEDOT:PSS/P3HT:PC61BM (1:08)/Al
- Short-circuit current density (Jsc): 9.5 mA/cm2
- Open-circuit voltage (Voc): 0.63 V
- Fill Factor (FF): 0.68
- Power Conversion Efficiency (PCE): 5 %
Autre catégorie plus écologique
Propriétés du semi-conducteur
P-type (mobility=1E-4-1E-1 cm2/V·s)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Caractéristiques et avantages
Good processibility, environmental stability and electroactivity.
Conditionnement
Informations légales
Rieke is a registered trademark of Rieke Metals, Inc.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Flexible electronic circuits and displays based on organic active materials are future generations of products that may eventually enter mainstream electronics market.
PCBM-based n-type semiconductors - Find p- and n-type organic semiconductors available with PCBM library & properties.
Flexible electronic circuits, displays, and sensors based on organic active materials will enable future generations of electronics products that may eventually enter the mainstream electronics market.
The application of conducting polymers at the interface with biology is an exciting new trend in organic electronics research.
-
For Product 445703, Poly(3-hexylthiophene-2,5-diyl), what does regioregular mean?
1 answer-
Helpful?
-
-
For Product 445703, Poly(3-hexylthiophene-2,5-diyl), what does "diyl" refer to?
1 answer-
The use of "diyl" is simply an extension of the use of the "yl" ending for a single substituent. For example, a monosubstituted ethane becomes ethyl - - - -, a monosubstituted propane becomes propyl - - - -, a monosubstituted butane becomes butyl - - - - and so on. Similarly, a 1,3-disubstituted butane becomes 1,3-butanediyl, indicating the presence of similar substituents on the 1- and 3- positions of the butane portion of the molecule.
Helpful?
-
-
For product 445703, Poly(3-hexylthiophene-2,5-diyl), what are the end groups for the polymer? What is the initiator for the polymerization?
1 answer-
For product 445703, Poly(3-hexylthiophene-2,5-diyl), one end group is a hydrogen and the other end group is a bromine. The name of the initiator for the polymerization is proprietary information.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What percentage is regioregular in Product 445703, Poly(3-hexylthiophene-2,5-diyl)?
1 answer-
Based on proton NMR, it is estimated to be approximately 98% regioregular.
Helpful?
-
-
What is the molecular weight of Product 445703, Poly(3-hexylthiophene-2,5-diyl)?
1 answer-
The molecular weight (MW) is approximately 87,000.
Helpful?
-
-
What is Product 445703, Poly(3-hexylthiophene-2,5-diyl), soluble in?
1 answer-
This product will be soluble in Carbon Tetrachloride, Chloroform, and Dichloromethane. It is also partially soluble in Tetrahydrofuran, ether and slightly soluble in Toluene.
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![[6,6]-Phenyl C61 butyric acid methyl ester ≥99%](/deepweb/assets/sigmaaldrich/product/structures/359/221/d990c746-0960-4c69-bf76-fe09b193824d/640/d990c746-0960-4c69-bf76-fe09b193824d.png)