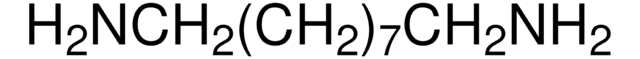213268
Calcium hydride
powder, 0-2 mm, reagent grade, ≥90% (gas-volumetric)
About This Item
Recommended Products
grade
reagent grade
Quality Level
Assay
≥90% (gas-volumetric)
form
powder
reaction suitability
reagent type: reductant
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
particle size
0-2 mm
mp
816 °C (lit.)
greener alternative category
SMILES string
[Ca]
InChI
1S/Ca.2H
InChI key
FAQLAUHZSGTTLN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
It may also be used as a reagent furing metallothermic synthesis of Nb3Al.
CaH2 can be used as a hydrogen source during the synthesis of monomethylated and dimethylated amines and in a ball milling process for the dechlorination of liquid and solid chlorinated compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Water-react 1
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service