All Photos(1)
About This Item
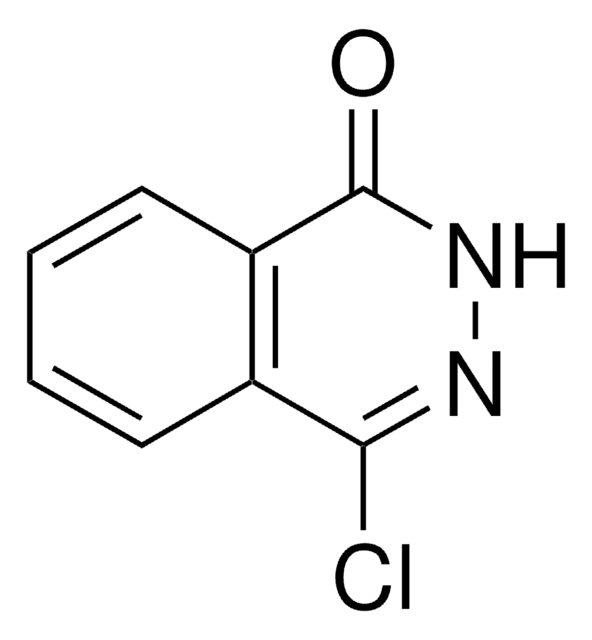
Empirical Formula (Hill Notation):
C14H10N2O
CAS Number:
Molecular Weight:
222.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
240-244 °C (lit.)
SMILES string
O=C1NN=C(c2ccccc2)c3ccccc13
InChI
1S/C14H10N2O/c17-14-12-9-5-4-8-11(12)13(15-16-14)10-6-2-1-3-7-10/h1-9H,(H,16,17)
InChI key
XCJLBNVENUPHEA-UHFFFAOYSA-N
General description
4-Phenyl-1-(2H)-phthalazinone can be prepared from hydrazine sulphate and sodium hydroxide.
Application
4-Phenyl-1-(2H)-phthalazinone may be used in the synthesis of 2-chloro-N-(unsubstituted/substitutedphenyl) acetamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kazuko Inoue et al.
Drug metabolism and disposition: the biological fate of chemicals, 42(8), 1326-1333 (2014-06-11)
Lenvatinib is a multityrosine kinase inhibitor that inhibits vascular endothelial growth factor receptors, and is being developed as an anticancer drug. P450s are involved in one of the elimination pathways of lenvatinib, and mono-oxidized metabolites, such as N-oxide (M3) and
Wagdy M Eldehna et al.
European journal of medicinal chemistry, 89, 549-560 (2014-12-03)
A novel series of N-substituted-4-phenylphthalazin-1-ones 14a-g bearing different anilines at the N-2 of phthalazin-1-one scaffold via acetyl-flexible linker was designed and synthesized for the development of potential anticancer agents. Compounds 19a-g were synthesized by insertion of methylene (CH2) bridge at
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service