All Photos(3)
About This Item
Empirical Formula (Hill Notation):
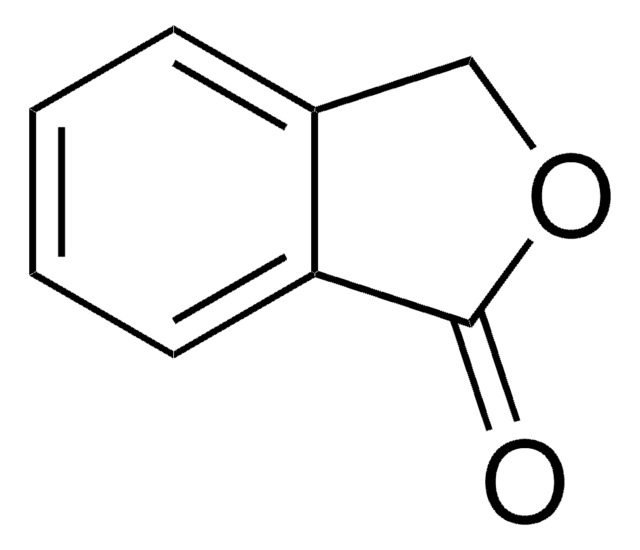
C10H8O3
CAS Number:
Molecular Weight:
176.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
111-113 °C (lit.)
SMILES string
OC(=O)C1CC(=O)c2ccccc12
InChI
1S/C10H8O3/c11-9-5-8(10(12)13)6-3-1-2-4-7(6)9/h1-4,8H,5H2,(H,12,13)
InChI key
HXLJFMRZKCSTQD-UHFFFAOYSA-N
General description
3-Oxo-1-indancarboxylic acid participates in the synthesis of 3-hydroxymethyl-1-indanol (±). Enantiomeric separation of 3-oxo-1-indancarboxylic acid on liquid chromatography chiral stationary phases (CSPs) by supercritical fluid chromatography (SFC) has been reported. Separation of 3-oxo-1-indancarboxylic acid enantiomers using a new immobilized polysaccharide chiral stationary phase, CHIRALPAK IA with hexane has been described.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Extending the Range of Solvents for Chiral Analysis Using a New Immobilized Polysaccharide Chiral Stationary Phase, CHIRALPAK IA.
Cox GB and Amoss CW.
LCGC North America, 22(2), 32-32 (2004)
Kinetic resolution of 3-hydroxymethylbenzocycloalkanols by selective asymmetric hydrogen-transfer oxidation.
Caro Y, et al.
Tetrahedron Asymmetry, 14(23), 3689-3696 (2003)
Super/subcritical fluid chromatography separations with four synthetic polymeric chiral stationary phases.
Han X, et al.
Chromatographia, 65(7-8), 381-400 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service