All Photos(1)
About This Item
Empirical Formula (Hill Notation):
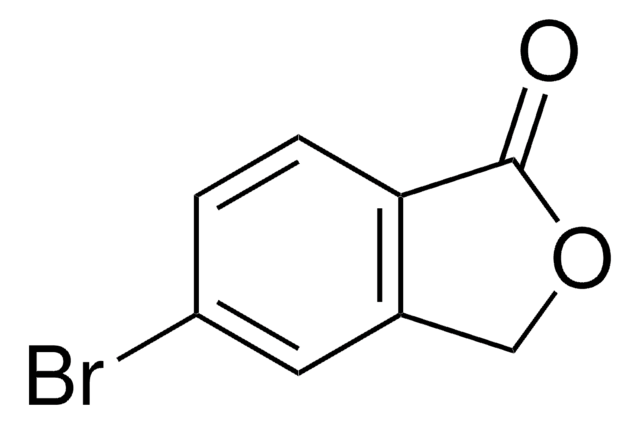
C8H6O2
CAS Number:
Molecular Weight:
134.13
Beilstein:
114632
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
bp
290 °C (lit.)
mp
71-74 °C (lit.)
SMILES string
O=C1OCc2ccccc12
InChI
1S/C8H6O2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2
InChI key
WNZQDUSMALZDQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
305.6 °F - closed cup
Flash Point(C)
152 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Juan Mangas-Sánchez et al.
Organic letters, 14(6), 1444-1447 (2012-03-08)
A straightforward synthesis of (S)-3-methylphthalides has been developed, with the key asymmetric step being the bioreduction of 2-acetylbenzonitriles. Enzymatic processes have been found to be highly dependent on the pH value, with acidic conditions being required to avoid undesired side
Zhishi Ye et al.
The Journal of organic chemistry, 75(17), 6043-6045 (2010-08-28)
A palladium-catalyzed addition of arylboronic acids to phthalaldehyde, followed by an intramolecular lactonization to access 3-substituted phthalides, is described. The procedure tolerates a series of functional groups, such as methoxyl, fluoro, chloro, and trifluoromethyl groups. It represents a procedure for
Tsz-Ying Yuen et al.
Organic letters, 14(19), 5154-5157 (2012-09-29)
A highly convergent total synthesis of 7',8'-dihydroaigialospirol is described. Key steps of the synthesis include a Nozaki-Hiyama-Kishi (NHK) coupling of an iodoalkyne with an advanced phthalide-aldehyde and a remarkable one-pot acid-mediated global deprotection/spiroacetalization.
Vatcharin Rukachaisirikul et al.
Journal of natural products, 75(5), 853-858 (2012-04-25)
Nine new fungal metabolites, one phthalide derivative, acremonide (1), and eight isocoumarin derivatives, acremonones A-H (2-9), were isolated from the mangrove-derived fungus Acremonium sp. PSU-MA70 together with 10 known compounds. Their structures were determined by NMR analysis. The known 8-deoxytrichothecin
R Santhosh Reddy et al.
Organic & biomolecular chemistry, 10(18), 3655-3661 (2012-04-13)
The asymmetric dihydroxylation (AD) of o-cyano cinnamates and styrene derivatives leads to efficient construction of chiral phthalide frameworks in high optical purities. This unique reaction is characterized by unusual synergism between CN and osmate groups resulting in rate enhancement of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service