All Photos(1)
About This Item
Empirical Formula (Hill Notation):
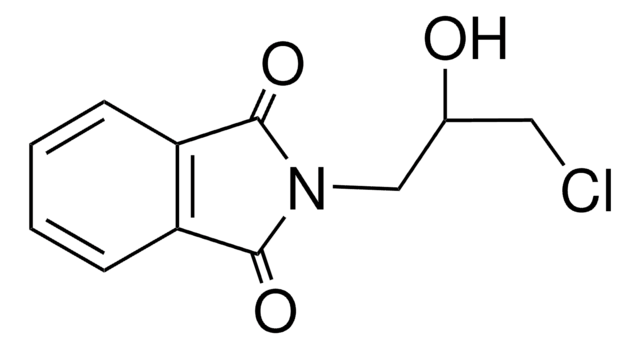
C11H11NO3
CAS Number:
Molecular Weight:
205.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
74-76 °C (lit.)
functional group
hydroxyl
imide
SMILES string
OCCCN1C(=O)c2ccccc2C1=O
InChI
1S/C11H11NO3/c13-7-3-6-12-10(14)8-4-1-2-5-9(8)11(12)15/h1-2,4-5,13H,3,6-7H2
InChI key
BSMILTTURCQDGJ-UHFFFAOYSA-N
General description
N-(3-Hydroxypropyl)phthalimide is ω-Hydroxyalkylphthalimide and is prepared by mixing phthalic anhydride and propanolamine and by heating at 160-180 °C for 4 hours.
Application
N-(3-Hydroxypropyl)phthalimide was used as standard in the synthesis of phthalimide derivatives at high-temperature, high-pressure and H2O/EtOH mixtures as the solvent. It may be used in the synthesis of hydrophilic phosphorylcholine-containing polymer, poly-2-[3-(methacryloylamino)propylammonio] ethyl 3-aminopropyl phosphate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rapid and clean synthesis of phthalimide derivatives in high-temperature, high-pressure H2O/EtOH mixtures.
Fraga-Dubreuil J, et al.
Green Chemistry, 9(10), 1067-1072 (2007)
Improvement of accuracy in flow immunosensor system by introduction of poly-2-[3-(methacryloylamino) propylammonio] ethyl 3-aminopropyl phosphate.
Fuchiwaki Y, et al.
Journal of Sensors null
Anna W Pierwocha et al.
Carbohydrate research, 343(15), 2680-2686 (2008-09-02)
We report a simple, efficient, and mild method for the synthesis of omega-aminoalkyl 2-deoxy-d-arabino/lyxo-hexopyranoside and 2,3-dideoxy-alpha-d-erythro-hexopyranoside. The total synthesis is accomplished in two sequential reactions. The first step consists of an addition reaction of N-(omega-hydroxyalkyl)phthalimide and N-(omega-hydroxyalkyl)succinimide to peracetylated d-glycals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service