All Photos(1)
About This Item
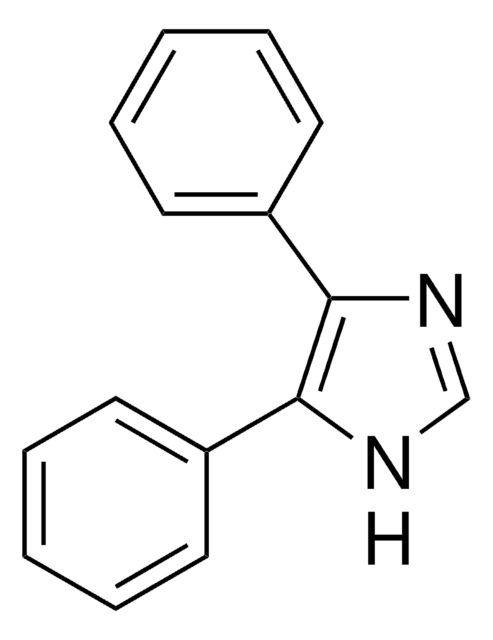
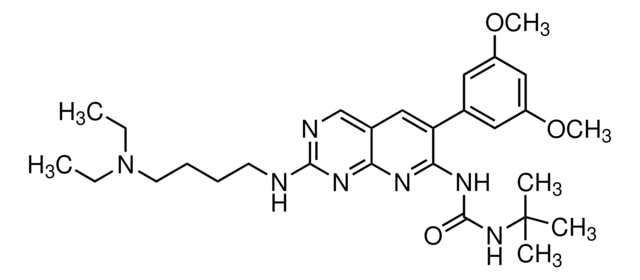
Empirical Formula (Hill Notation):
C13H10N2
CAS Number:
Molecular Weight:
194.23
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
293-296 °C (lit.)
SMILES string
c1ccc(cc1)-c2nc3ccccc3[nH]2
InChI
1S/C13H10N2/c1-2-6-10(7-3-1)13-14-11-8-4-5-9-12(11)15-13/h1-9H,(H,14,15)
InChI key
DWYHDSLIWMUSOO-UHFFFAOYSA-N
Gene Information
human ... FGFR1(2260) , PDGFRB(5159)
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The interaction of 2-phenylbenzimidazole compounds with DNA: the influence of terminal substituents.
Yukio Kubota et al.
Nucleic acids research. Supplement (2001), (2)(2), 193-194 (2003-08-09)
We have studied the influence of terminal substituents, amidinium group and N-methylpiperazine ring, of 2-phenylbenzimidazole compounds (1-6; Figure 1) on their DNA-binding modes. Experimental results reveal that 1-3 are accepted in intercalation pockets owing to structural flexibility of the N-methylpiperazine
Michele Tonelli et al.
Bioorganic & medicinal chemistry, 18(6), 2304-2316 (2010-03-02)
Starting from a series of our new 2-phenylbenzimidazole derivatives, shown to be selectively and potently active against the bovine viral diarrhea virus (BVDV), we developed a hierarchical combined experimental/molecular modeling strategy to explore the drug leads for the BVDV RNA-dependent
Mehrorang Ghaedi et al.
Journal of hazardous materials, 158(1), 131-136 (2008-03-04)
A new and efficient solid phase extraction method is described for the preconcentration of trace heavy metal ions. The method is based on the adsorption of Fe(3+), Cu(2+) and Zn(2+) on 2-phenyl-1H-benzo[d] imidazole (PHBI) loaded on Triton X-100-coated polyvinyl chloride
J Johnson Inbaraj et al.
Photochemistry and photobiology, 75(2), 107-116 (2002-03-09)
The sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid (PBSA) and its parent 2-phenylbenzimidazole (PBI) cause DNA photodamage via both Type-I and Type-II mechanisms when UVB irradiated. We have studied the photophysical and photochemical properties of these compounds and their ability to photogenerate reactive
Mark L Richards et al.
European journal of medicinal chemistry, 41(8), 950-969 (2006-05-02)
The pharmacotherapy of allergy and asthma has traditionally focused on the effecter molecules of the allergic cascade, while neglecting targets that play an early role in their development. Reasoning that IgE is central to the expansion of atopic diseases, we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service