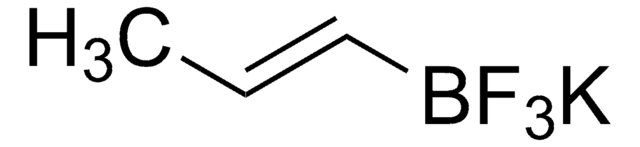
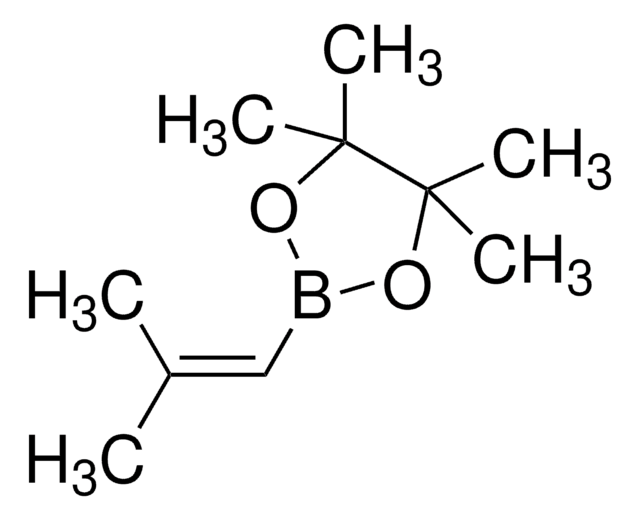
576638
trans-1-Propen-1-ylboronic acid
≥95.0%
Synonym(s):
(E)-1-Propen-1-ylboronic acid, (E)-Prop-1-enylboronic acid, trans-1-Propeneboronic acid, trans-1-Propenylboronic acid, trans-Propenylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH=CHB(OH)2
CAS Number:
Molecular Weight:
85.90
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
impurities
~10 wt. % cis-isomer
mp
123-127 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(C)=C(\[H])B(O)O
InChI
1S/C3H7BO2/c1-2-3-4(5)6/h2-3,5-6H,1H3/b3-2+
InChI key
CBMCZKMIOZYAHS-NSCUHMNNSA-N
Application
Reactant for:
Reactant for preparation of:
- Palladium-phosphine-catalyzed Suzuki-Miyaura coupling reactions
- Cu(II)-mediated Ullmann-type coupling
Reactant for preparation of:
- Alkynylphenoxyacetic acids as DP2 receptor antagonists for treatment of allergic inflammatory diseases
- Tetrahydrobenzothiophenes as conformationally restricted enol-mimic inhibitors of type II dehydroquinase via Paal-Knorr synthesis involving Suzuki coupling
- Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-mediated cyclization
- Stereospecific dienes via nickel-catalyzed three-component reductive coupling with alkynes and enones
Reactant for
Reactant for preparation of
- Palladium-phosphine-catalyzed Suzuki-Miyaura coupling reactions
- Cu(II)-mediated Ullmann-type coupling
- Palladium-catalyzed Sonogashira cross-coupling
Reactant for preparation of
- Alkynylphenoxyacetic acids as DP2 receptor antagonists for treatment of allergic inflammatory diseases
- Tetrahydrobenzothiophenes as conformationally restricted enol-mimic inhibitors of type II dehydroquinase via Paal-Knorr synthesis involving Suzuki coupling
- Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-mediated cyclization
- Stereospecific dienes via nickel-catalyzed three-component reductive coupling with alkynes and enones
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A new strategy for the synthesis of substituted dihydropyrones and tetrahydropyrones via palladium-catalyzed coupling of thioesters
Fuwa, H.; et al.
Tetrahedron, 67, 4995-5010 (2011)
Ming-Bo Zhou et al.
The Journal of organic chemistry, 75(16), 5635-5642 (2010-08-14)
Palladium-catalyzed cross-coupling reaction of terminal alkynes with arylboronic acids has been described. In the presence of Pd(OAc)(2) and Ag(2)O, a variety of terminal alkynes, including electron-poor terminal alkynes, smoothly underwent the reaction with numerous boronic acids to afford the corresponding
Total synthesis of rodgersinol: a survey of the Cu(II)-mediated coupling of ortho-substituted phenols
Jung, J.-W.; et al.
Tetrahedron, 66, 6826-6831 (2010)
Chun-Ming Yang et al.
Organic letters, 12(16), 3610-3613 (2010-08-14)
A highly regio- and stereoselective nickel-catalyzed three-component coupling of alkynes with enones and alkenyl boronic acids to afford highly substituted 1,3-dienes is described. The reaction can also be extended to cyclization of enynes with coupling to alkenyl boronic acids. A
Daohong Yu et al.
The Journal of organic chemistry, 77(4), 1798-1804 (2012-01-31)
A selective palladium-catalyzed Suzuki-Miyaura coupling reaction of polyfluorophenyl oxazolines through ortho C-F activation is described. It was found that reactions with DPPF as the ligand occurred much faster than those with other ligands. A variety of arylboronic acids including challenging
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service