Chemically Defined Media Supplements Improve Quality and Lower Risk in Upstream Bioprocessing
Hydrolysates are commonly used in cell culture media as a supplement to enhance cell growth and recombinant protein productivity. They contain diverse classes of compounds, such as peptides, carbohydrates and phenolic compounds. However, these naturally derived hydrolysates are undefined. As an alternative, chemically defined (CD) supplements can be used.
Why Use a Chemically Defined Media Supplement for Bioprocessing?
There are many reasons to use a chemically defined media supplement in bioprocessing including:
- Reduce lot-to-lot variation
- Lower risk of contamination
- Facilitate favorable product quality attributes (ex: glycosylation)
As a case study, we’ll take a look at how we created the chemically defined supplement EX-CELL® CD Hydrolysate Fusion and its performance in mAb-producing CHO cell lines.
Developing a Chemically Defined Alternative to Natural Hydrolysates for CHO Cell Cultures
To develop this chemically defined alternative, various natural hydrolysates were fractionated by reversed phase HPLC; fractions were screened in CHO cell cultures with a base supplement that contains easily identified hydrolysate components. The fractions which had a significant impact on the cultures were identified and chemically defined versions were sourced. The chemically defined alternatives were screened with multiple CHO cell lines and a final combination was created with the base supplement, which is now available as EX-CELL® CD Hydrolysate Fusion.
Cultures grown using EX-CELL® CD Hydrolysate Fusion were comparable to cultures grown using the undefined, natural product. Viable cell density and titer were comparable in a batch process (Figure 1). We saw similar results with a different CHO clone in a separate batch study (Figure 2). These data demonstrate that the EX-CELL® CD Hydrolysate Fusion is a good alternative to replace undefined hydrolysates while maintaining or achieving higher titers.
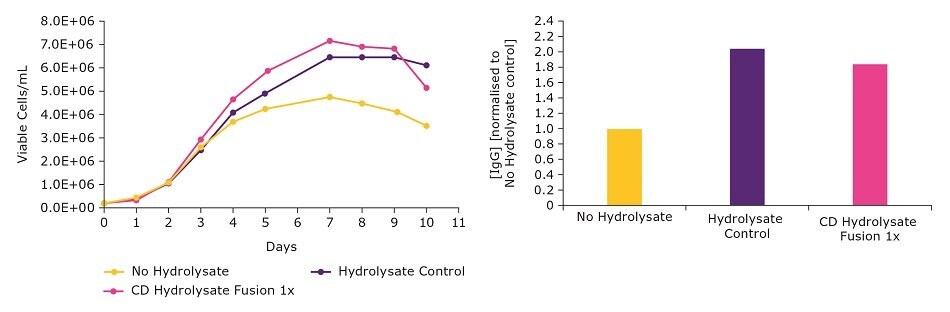
Figure 1.Comparison of chemically defined and natural hydrolysates on viable cell density and productivity in a batch process.
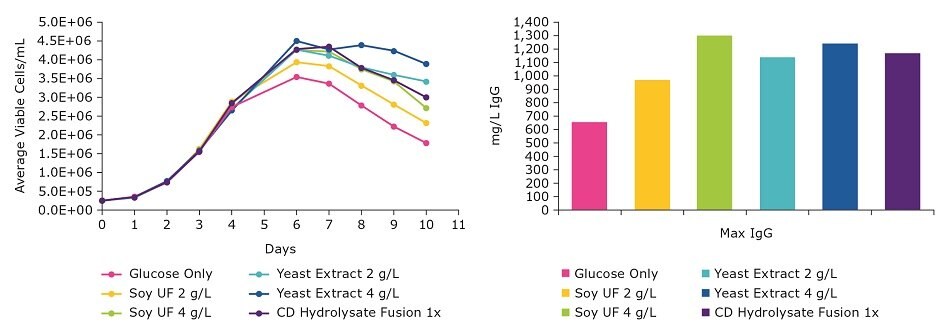
Figure 2.Comparison of EX-CELL® CD Hydrolysate Fusion and natural hydrolysates in a batch process.
Using Chemically Defined Supplements to Impact Glycosylation
Chemically defined supplements can also be used to facilitate favorable product quality attributes such as glycosylation which affects the structure, folding ability, stability, in vivo half-life, immunogenicity, and efficacy of a drug molecule. One way to rapidly modify glycan profiles is to use a targeted protein quality supplement. The specific purpose of said supplement is to increase galactose site occupancy in order to achieve functional shifts in N-linked glycosylation quickly and efficiently.
We conducted two experiments to illustrate how a supplement can help to shift glycan profiles.
First, we examined fed-batch cultures in spin tubes with three different mAb-producing CHO cell lines (CHO-M, DuxB11, and CHOZN® GS) cultured with and without the EX-CELL® Glycosylation Adjust supplement (GAL+). Then, we evaluated scalability of supplementation with GAL+.
We found a two-to-four fold increase in the relative G1F and G2F distribution both in the fed-batch culture in spin tubes, across the range of CHO cell lines, and during the subsequent scale up of the CHOZN® GS cell line to the 3 L Mobius® bioreactor; all three CHO cell lines showed a similar shift towards G1F and G2F (Figure 3). There was no change in growth and titer profiles (data not shown).
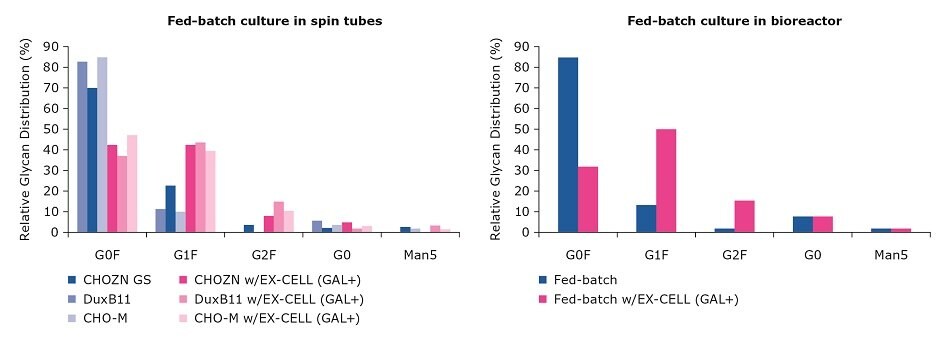
Figure 3.Change in relative G1F and G2F distribution using the GAL+ supplement.
Changing parameters such as temperature, pH, and dissolved oxygen can optimize titer yields, but these changes also affect glycan profiles. In the following example, a temperature shift to 34 °C was initiated on day 6 and continued to day 14. While there was an increase in titer from 1.5 g/L to 2 g/L, the process resulted in an increase of G0F. With GAL+ supplementation, the glycan profiles were shifted without affecting the titer improvement achieved with the earlier process optimization. This study confirmed that GAL+ supplement can be used together with temperature shift to achieve an increase in titer, G1F, and G2F.
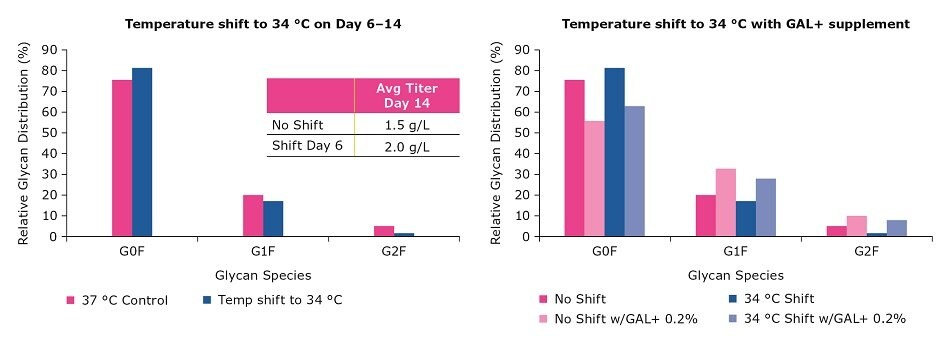
Figure 4.Use of GAL+ supplement to balance titer and glycosylation requirements.
Conclusion
The ability to optimize scalable upstream processes sets the stage for successful clinical- and commercial-scale production. A chemically defined hydrolysate can yield equivalent performance while reducing the risk in raw materials, lot-to-lot variation, process variations, and possible contamination. Use of a novel targeted protein quality supplement increases galactose site occupancy, modifying N-glycan profiles across a broad range of CHO cell lines.
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?