What is an Antibody-Drug Conjugate?
Antibody-drug conjugates (ADCs) are a fast-growing drug modality primarily used as a cancer therapeutic. As of early 2023, there are over 213 ADCs in clinical development and 13 ADCs approved by the FDA.1
What is an Antibody-Drug Conjugate?
An ADC is composed of a monoclonal antibody conjugated to a drug payload via a linker. This complex targets the drug to a specific cell type (e.g.: cancer cell) for therapeutic effect. The selectivity of the mAb affects the cancer cell while sparing healthy cells.
Compared to traditional chemotherapy, ADCs have many advantages, including:
- Tumor selectivity
- Longer half life
- Improved safety profiles (while still highly potent, they exhibit reduced systemic toxicity)
Components of an Antibody-Drug Conjugate
There are three primary components of an ADC: the antibody, the payload, and the linker (Figure 1).
- Monoclonal antibody: The antibody used in ADCs should be specific for a tumor-associated antigen that has restricted expression on normal cells. Most antibodies used are either humanized or human monoclonal antibodies.2
- Linker: The linker attaches the payload (a cytotoxic agent) to the antibody. It is designed to be stable in circulation and release the payload once inside targeted cells. Linkers can have many characteristics (e.g. cleavable vs. non-cleavable) and can affect ADC solubility, stability, toxicity, and other factors.
- Payload: The payload is designed to kill target cells when internalized and released. These payloads are often drugs that are highly toxic and require antibodies to specifically target cancer cells so they do not affect healthy cells. Payload synthesis is a complex process, but use of ADC payload intermediates can expedite and simplify access to highly potent APIs (HPAPIs).
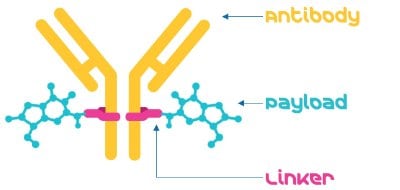
Figure 1.An Antibody-drug conjugate consists of an antibody, a linker, and a payload.
How do Antibody-Drug Conjugates work?
For the majority of ADCs with cytotoxic payloads, a cascade of steps lead to killing a cancer cell (Figure 2):
- Binding: The antibody portion of the ADC binds the antigen on the cancer cell.
- Uptake: The ADC is internalized into the cell and enters the endosome-lysosome pathway.
- Antibody degradation: The ADC is degraded by the lysosome, separating the payload from the now degraded antibody and linker.
- Payload release: The toxic payload is released from the lysosome into the cancer cell’s cytoplasm/nucleus.
- DNA or microtubule disruption: Once released, the payload reaches its intercellular target (e.gdisrupting the cancer cell’s DNA or microtubules).
- Cell death: The payload’s mechanism of action causes cancer cell death.
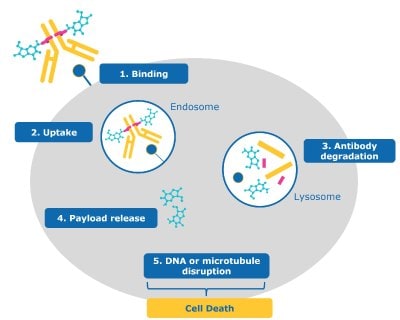
Figure 2.ADC mechanism of action for cancel cell targeting and killing (1) binding, (2) uptake, (3) antibody degradation, (4) payload release, (5) DNA or microtubule disruption, and (6) cell death.
ADC Design and Development Considerations
Because ADCs comprise multiple components that have varying effects on the ADC, their design is not trivial. In addition to optimizing mAb, payload, and linker, their conjugation presents additional considerations:
- Site of Conjugation (stochastic versus site-specific)
- Effects of conjugation on mAb: Changes to the mAb can affect its biological properties such as stability or targeting effectiveness. It’s important to consider these effects when choosing the linker and payload.
- Drug-to-antibody ratio optimization: The drug-to-antibody ratio (DAR) is the average number of drug molecules conjugated to an antibody. A low DAR could reduce the effectiveness of the ADC, but a high DAR could negatively impact antibody structure, stability, or antibody binding.
- Payload aggregation: Some payloads, such as pyrrolobenzodiazepine are hydrophobic and can cause the ADC to aggregate. Sometimes integration of a solubilizer will resolve, or even improve, ADC solubility and efficacy.
- Purification after antibody-drug conjugation reaction: The conjugation reaction results in a mixture of ADCs with DARs, free drug and linker molecules, and various solvents that need to be removed.
ADC Contract Development and Manufacturing Considerations
Due to the complexities and containment handling to requirements of ADCs, many developers companies turn to CTDMOs (Contract Testing, Development and Manufacturing organizations) or CDMOs (Contract Development, Manufacturing Organizations) to develop, manufacture, and test ADCs.
CTDMOs, offering end-to-end services to bring ADCs and bioconjugates into the clinic and to commercialization can be the preferred option when looking for an integrated and reliable supply chain from cell line development to testing of bulk drug substance. Adding expertise in regulatory support for seamless scale-up (IND, PPQ, BLA/NDA) and innovative technologies to advance drug discovery and development.
For developers interested in generating ADCs but require expertise or conjugation capabilities, our ADC Express Services™ leverage our extensive bioconjugation experience to provide libraries of development-grade ADCs for best candidate selection.
Next-Generation Bioconjugates and the Future of ADCs
While traditional ADCs use a monoclonal antibody paired with a pan-cytotoxic small molecule, next-generation bioconjugates have adopted the bioconjugate model for pairing various antibody formats (ex: fragment antigen-binding region (Fab region), single-chain variable fragments (scFv), and nanobodies) with diverse payloads (e.g.: small molecules, radio-nuclides, proteins, and oligonucleotides) (Figure 3). Such novel approaches to bioconjugates opens doors to new mechanisms of action, therapies, and indications.
Traditional

Figure 3a.mAb as component of an adc or bioconjugate
Figure 3b.Payload, or pan-cytotoxic small molecule as component of an adc or bioconjugate.
Emerging

Figure 3c.Bispecific antibody and fragment-based bi-/multispecific constructs for payload delivery.
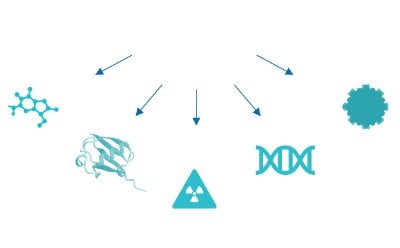
Figure 3d.Small molecule, protein, radio nuclide, oligonucleotide, nanoparticle.
Take the next step towards bringing your project to market quickly and safely by speaking to one of our technical experts.
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?