Greener Methods: Catalytic Amide Bond Formation
Introduction
Amide bonds are one of the most frequently utilized disconnections in organic synthesis. Methods to form this vital functionality are commonly superstoichiometric, leading to considerable waste in the process. These facts have led the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable (ACS GCIPR) to identify the catalytic formation of amide bonds as a key initiative for Green Chemistry.1
We are proud to offer a number of products used in catalytic amidation technology.2
Representative Transformations
In 2007, Milstein and coworkers reported the ruthenium-catalyzed dehydrogenative coupling of amines and alcohols to give amide products.3
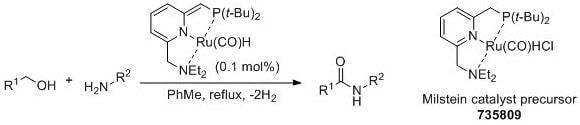
Madsen and coworkers later reported an alternate catalyst system for this dehydrogenative coupling with broader scope.4
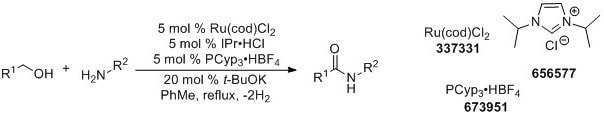
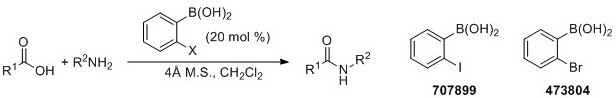
More recently, Hall and coworkers have demonstrated boronic acid catalyzed amidations using carboxylic acids as substrates.5
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?