Bis(pyridine)iodonium Tetrafluoroborate Usage
(Ipy2BF4, Barluenga’s Reagent)
This mild iodinating and oxidizing reagent is capable of selectively reacting with a wide range of unsaturated substrates and tolerates a variety of functional groups. Ipy2BF4 reacts with acetonides derived from simple terpenes to accomplish selective iodofunctionalization with excellent regio- and diastereofacial control (Scheme 25).1

Scheme 25.
When added to 2-alkynyl-substituted benzaldehydes, Ipy2BF4 produces substituted naphthalene products upon treatment with either an alkene or alkyne (Scheme 26).2 Alternatively, subsequent treatment with primary alcohols affords oxygen containing heterocycles (Scheme 27).3
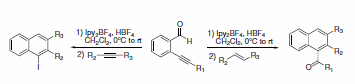
Scheme 26.
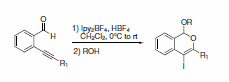
Scheme 27.
Recently, tetracyclic tetrahydrofurans were produced using Ipy2BF4 in a key step of the synthesis of a series of potential broad- spectrum psychotropic agents (Scheme 28).4
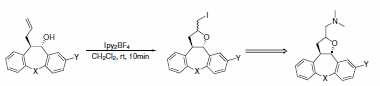
Scheme 28.
Additionally, Ipy2BF4 has been reported to be useful in general iodinations (Scheme 29)5 and oxidation of alcohols to carbonyls.6 Photolytic reactions of cycloalkanols produced ring-cleaved products, while thermal reactions left the ring intact during oxidation (Scheme 30). Thermal reactions of primary alcohols were capable of producing either aldehydes in dilute conditions or esters in more concentrated solutions (Scheme 31).
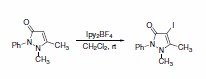
Scheme 29.
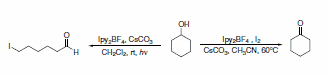
Scheme 30.
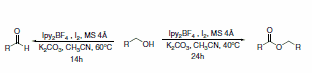
Scheme 31.
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?