Sildenafil Citrate USP Method Using HPTLC Plates for the Limit of Imidazole Test
Sanjay Poman
Introduction
Sildenafil, sold under the brand name Viagra, among others, is a medication that can be used as a selective inhibitor of cyclic GMP- specific photodiesterase (type V) with vasodilator action. It can be used to treat erectile dysfunction and pulmonary arterial hypertension. In 1994, Pfizer filed a patent covering the use of sildenafil to treat erectile dysfunction. This patent will expire in 2019.
In the present study we demonstrate compliance of silica gel 60 F254 HPTLC plates with USP requirements, and how HPTLC plates are suitable for the Sildenafil citrate- Limit of Imidazole test.

Figure 1.5-[2-ethoxy-5-(4-methylpiperazin-1-yl)sulfonylphenyl]-1-methyl-3-propyl-4H-pyrazolo[4,3-d]pyrimidin-7-one
| Experimental Conditions | |
|---|---|
| TLC Plate | PTLC Silica Gel 60 F254 20 x 10 cm |
| Detection | Expose to iodine vapors until the plate is light brown & examine the plate in ultra violet light at 254 nm |
| Mobile Phase | Methylene chloride:ethyl acetate:alcohol:ammonium hydroxide (50:30:20:1 v/v/v) |
| Plate Development | Examine under ultra violet light at 254 nm |
| Development | Over 2/3rd of the plate |
| Diluent | Methanol:water:ammonium hydroxide (15:5:1 v/v/v) |
| Drying | At 100 °C for 15 minutes |
| Standard | a) Dissolve 0.035 mg/ml of imidazole in diluent b) Dissolve 0.0175 mg/ml of imidazole in reference solution a SST Solution:- Mix equal volume of sample solution & standard solution a |
| Sample | Dissolve 17.5 mg of sildenafil citrate in diluent |
| Injection Volume | 10 µL (6 mm band) |
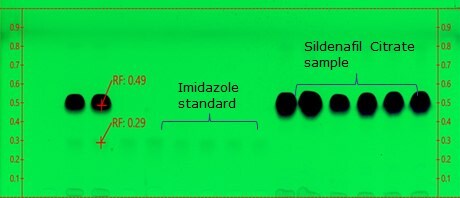
Figure 2.TLC Plate after development
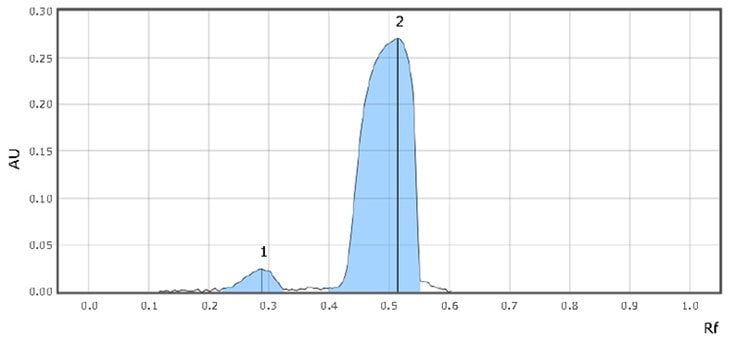
Figure 3.System suitability (SST) chromatograms
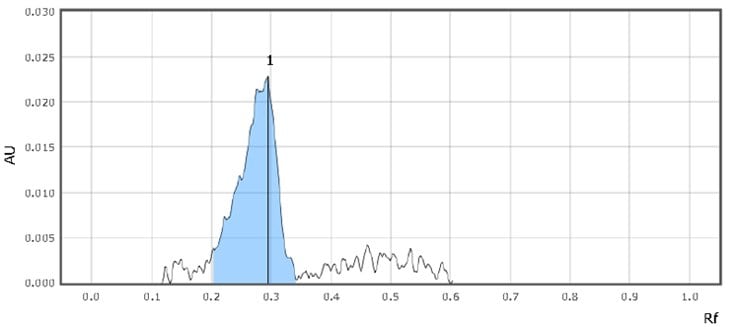
Figure 4.Chromatographic Data (Standard Solution)
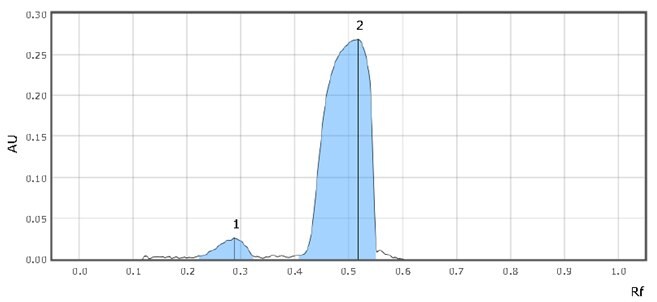
Figure 5.Chromatographic Data (SST Solution)
| Compound | Rf | Area | |
|---|---|---|---|
| 1 | Imidazole | 0.3 | 160 |
| Compound | Rf | Area | |
|---|---|---|---|
| 1 | Imidazole | 0.3 | 143 |
| 2 | Sildenafil citrate | 0.5 | 2669 |
Summary
- The principal spot in the chromatogram obtained with the test solution is not more intense than the principal spot from standard solution b, as expressed in the monograph acceptance criteria.
- Retardation factor (Rf) for imidazole and sildenafil is 0.3 & 0.5, respectively. The chromatogram should show two clearly separated zones, with Rf-values of about 0.25 and 0.4 for imidazole and sildenafil citrate, respectively (suitability requirements section).
- The silica gel HPTLC plates, combined with ethyl acetate and methanol of Lichrosolv quality provide reproducible results for the USP monograph method Sildenafil Citrate - limit of imidazole test and comply overall with USP requirements.
Network error: Failed to fetch
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?