14-326-M
Src Protein, active, 10 µg
Active, N-Terminal His6-tagged, recombinant, full-length, human Src. For use in Kinase Assays.
Synonym(s):
SRC1
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352202
eCl@ss:
32160405
NACRES:
NA.26
Recommended Products
biological source
human
Quality Level
recombinant
expressed in Sf21 cells
form
liquid
mol wt
Mw 61.7 kDa
manufacturer/tradename
Upstate®
technique(s)
activity assay: suitable (kinase)
solubility
soluble
NCBI accession no.
UniProt accession no.
Gene Information
human ... SRC(6714)
General description
Research area: Cell Signaling
N-Terminal His6-tagged, recombinant, full-length, human Src.
Src proteins are non-receptor protein tyrosine kinases, that belong to the Src family of kinases. These proteins have a unique structure consisting of an N-terminal region with a 14-carbon myristoyl group, two Src homology domains (SH2 and SH3), a catalytic tyrosine-protein kinase domain (SH1), and a short C-terminal tail. The SH1 domain is catalytic in function, while the SH2 and SH3 domains are non-catalytic and regulatory in nature.
Product Source: Human c-src, expressed in Sf21 cells.
N-Terminal His6-tagged, recombinant, full-length, human Src.
Src proteins are non-receptor protein tyrosine kinases, that belong to the Src family of kinases. These proteins have a unique structure consisting of an N-terminal region with a 14-carbon myristoyl group, two Src homology domains (SH2 and SH3), a catalytic tyrosine-protein kinase domain (SH1), and a short C-terminal tail. The SH1 domain is catalytic in function, while the SH2 and SH3 domains are non-catalytic and regulatory in nature.
Product Source: Human c-src, expressed in Sf21 cells.
Application
The Src protein may be used to study the interaction between CD133 and Src protein and its effect on FAK phosphorylation and cell migration through in vitro kinase assay.
Biochem/physiol Actions
Src protein tyrosine kinases are involved in signaling pathways that control a plethora of cellular functions ranging from cell cycle, proliferation, differentiation, gene transcription, immune responses, apoptosis, etc. Upon its activation, Src phosphorylates and activates several other kinases Like MAPK (Mitogen-Activated Protein Kinase), p38, and ERK (Extracellular signal-regulated kinase). Activated MAPK (Mitogen-Activated Protein Kinase) regulates inflammation, cell development, differentiation and senescence. P38 is involved with the migration and survival of endothelial cells and extracellular signal-regulated kinase (ERK) promotes the proliferation and inflammation of endothelial cells. They play a key role in the regulation of cytoskeletal organization through the phosphorylation of substrates like Actin filament-associated protein 1 (AFAP1) and cortactin (CTTN). Src also plays a crucial role in maintaining cell adhesion, morphology, motility, and bone resorption by interacting with focal adhesion kinases. Src tyrosine kinase activity is upregulated in a variety of cancers including colon, lung, and pancreatic cancer, etc.
Packaging
2 vials at 5μg each
Also available in 250μg size (2x125μg)--call for pricing and availability and reference catalog number 14-326M when ordering the 250μg size.
Also available in 250μg size (2x125μg)--call for pricing and availability and reference catalog number 14-326M when ordering the 250μg size.
Quality
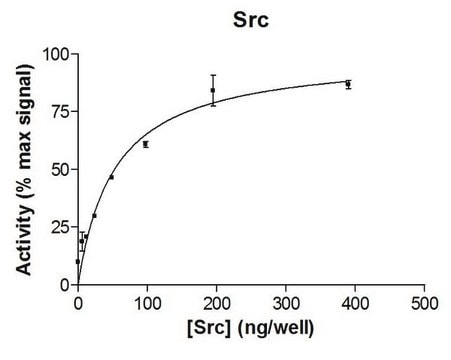
Routinely evaluated by phosphorylation of Src Substrate Peptide
Other Notes
For Specific Activity data, refer to the Certificate of Analysis for individual lots of this enzyme.
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protein tyrosine kinases, with emphasis on the Src family.
Courtneidge, S A
Seminars in Cancer Biology, 5, 239-246 (1994)
Jianhui Ma et al.
Cancer cell, 35(3), 504-518 (2019-03-05)
Ionizing radiation (IR) and chemotherapy are standard-of-care treatments for glioblastoma (GBM) patients and both result in DNA damage, however, the clinical efficacy is limited due to therapeutic resistance. We identified a mechanism of such resistance mediated by phosphorylation of PTEN
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service