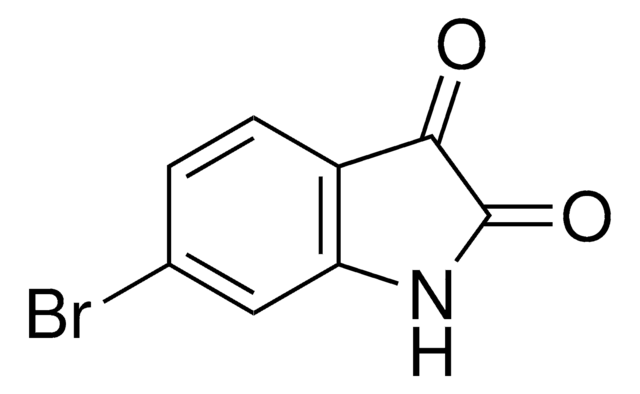
377406
3,4-Dimethoxy-3-cyclobutene-1,2-dione
99%
Synonym(s):
Dimethyl squarate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)2C4(=O)2
CAS Number:
Molecular Weight:
142.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
55-57 °C (lit.)
functional group
ether
ketone
storage temp.
2-8°C
SMILES string
COC1=C(OC)C(=O)C1=O
InChI
1S/C6H6O4/c1-9-5-3(7)4(8)6(5)10-2/h1-2H3
InChI key
SZBNZTGCAMLMJY-UHFFFAOYSA-N
Related Categories
General description
3,4-Dimethoxy-3-cyclobutene-1,2-dione (Dimethyl squarate) is a cyclobutene derivative. It reacts with hydroxylamine derivatives to afford 3-hydroxyamino-4-methoxy-3-cyclobutene-1,2-diones.
Application
3,4-Dimethoxy-3-cyclobutene-1,2-dione (Dimethyl squarate) may be used in the synthesis of the following compounds:
- chiral squaramides, highly enantioselective catalyst for the Friedel-Crafts reactions of indoles
- 3-(hydroxyamino)-4-methoxy-3-cyclobutene-1,2-dione
- squarate derivatives of the O-SP-core antigens
- methyl squarate derivative of the Ogawa O-SP-core antigen
- o-quinodimethanes
- benzocyclobutenes
- quinones
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mohammad Murshid Alam et al.
PLoS neglected tropical diseases, 8(2), e2683-e2683 (2014-02-12)
Protective immunity against cholera is serogroup specific. Serogroup specificity in Vibrio cholerae is determined by the O-specific polysaccharide (OSP) of lipopolysaccharide (LPS). Generally, polysaccharides are poorly immunogenic, especially in young children. Here we report the evaluation in mice of a
The Journal of Organic Chemistry, 59, 3284-3284 (1994)
Yong Qian et al.
Chemical communications (Cambridge, England), 46(17), 3004-3006 (2010-04-14)
Chiral squaramides are highly enantioselective catalysts for Friedel-Crafts reaction of indoles with N-tosyl imines, affording 3-indolyl methanamine products in 85-96% yields and 84-96% ees.
Tetrahedron Letters, 34, 6177-6177 (1993)
Hydroxylamin-Derivate der Quadratsaure.
Zinner G, et al.
Arch. Pharm. (Weinheim), 318(11), 977-983 (1985)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service