127574
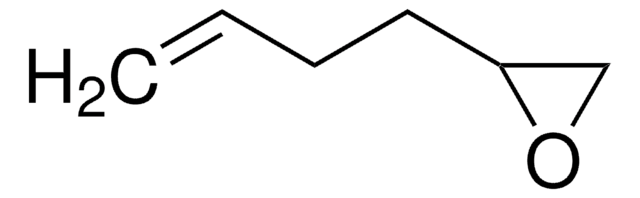
3,4-Epoxy-1-butene
98%
Synonym(s):
2-Vinyloxirane, 3,4-Epoxy-1-butene, Butadiene monoxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O
CAS Number:
Molecular Weight:
70.09
Beilstein:
103170
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
65-66 °C (lit.)
density
0.87 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C=CC1CO1
InChI
1S/C4H6O/c1-2-4-3-5-4/h2,4H,1,3H2
InChI key
GXBYFVGCMPJVJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-58.0 °F - closed cup
Flash Point(C)
-50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eduardo Cemeli et al.
Mutation research, 664(1-2), 69-76 (2009-05-12)
The toxicity of butadiene and styrene is exerted by their metabolites. Such metabolites have been extensively scrutinized at the in vitro level demonstrating evident genotoxic properties. In monitoring, a diverse range of outcomes has been produced. Additionally, epidemiological studies in
Stereoselective ring expansion of vinyl oxiranes: mechanistic insights and natural product total synthesis.
Matthew Brichacek et al.
Angewandte Chemie (International ed. in English), 49(9), 1648-1651 (2010-02-06)
L Recio et al.
Chemico-biological interactions, 135-136, 325-341 (2001-06-09)
1,3-Butadiene (BD) is a multisite carcinogen and is mutagenic in multiple tissues of B6C3F1 mice. BD is bioactivated to at least three directly mutagenic metabolites: 1,2-epoxybutene (EB), 1,2-epoxy-3,4-butanediol (EBD), and 1,2,3,4-diepoxybutane (DEB). However, the contribution of these individual metabolites to
Some insights into the mode of action of butadiene by examining the genotoxicity of its metabolites.
A D Kligerman et al.
Chemico-biological interactions, 166(1-3), 132-139 (2006-05-16)
1,3-Butadiene (BTD) is an important commodity chemical and air pollutant that has been shown to be a potent carcinogen in mice, and to a lesser extent, a carcinogen in rats. To better assess butadiene's carcinogenic risk to humans, it is
Thomas J L Mustard et al.
Journal of the American Chemical Society, 135(4), 1471-1475 (2013-01-01)
Density functional theory computations of the Cu-catalyzed ring expansion of vinyloxiranes is mediated by a traceless dual Cu(I)-catalyst mechanism. Overall, the reaction involves a monomeric Cu(I)-catalyst, but a single key step, the Cu migration, requires two Cu(I)-catalysts for the transformation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service