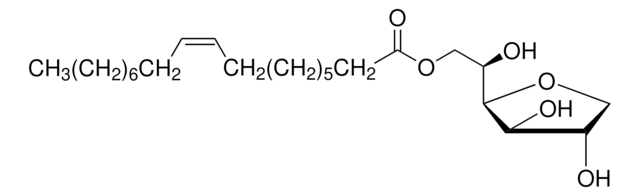
This product requires dilution with saline prior to use. This dilution can be done with or without the addition of the antigen. See below for a brief protocol.
Prior to antigen addition, warm contents of vial to 40-45 °C. Add 2.0 mL of sterile saline containing the desired amount of antigen.
The final emulsion will contain a concentration of 2% oil.
1. Inject antigen-saline solution (2 mL) directly into the vial through the rubber stopper using a syringe fitted with a 20 or 21-gauge needle (leave the cap seal in place).
2. Vortex the vial vigorously for 2 to 3 minutes to form emulsion. Invert the vial and vortex for 1 minute to ensure reconstitution of any product adhering to the stopper.
3. If the entire contents of the vial will not be used initially, reconstitute with 1 ml saline without antigen. This emulsion can be stored at 2-8 °C for up to 60 days. Do Not freeze.
To use, mix aliquots 1:1 with antigen in saline.
4. Prior to inoculation, warm the vial to 37 °C and vortex briefly.
For full details on injection protocols and boosting, please navigate to the link https://www.sigmaaldrich.com/techservice, click on "Product Technical Inquiries" under the Products Section with all the required information so that a member of the Technical Service team can reach out to assist further. Thank you.