Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
N7634
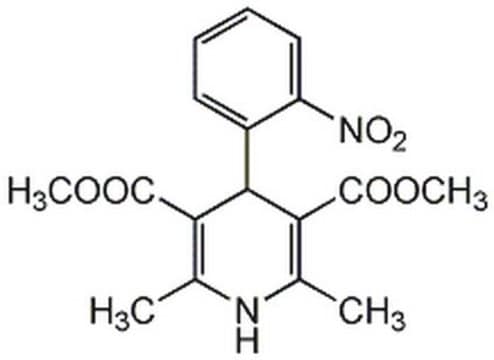
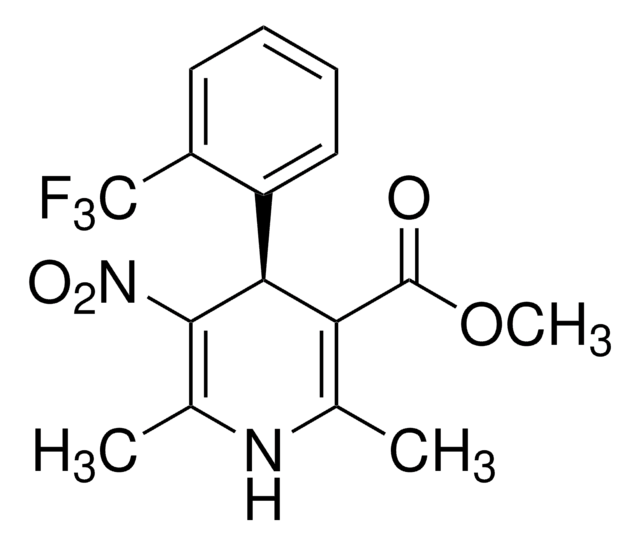
Nifedipine
≥98% (HPLC), powder, L-type Ca²⁺ channel blocker
Synonyme(s) :
1,4-Dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid dimethyl ester
Sélectionner une taille de conditionnement
75,90 €
Sélectionner une taille de conditionnement
About This Item
75,90 €
Produits recommandés
Nom du produit
Nifedipine, ≥98% (HPLC), powder
Niveau de qualité
Essai
≥98% (HPLC)
Forme
powder
Couleur
yellow
Solubilité
DMSO: soluble
ethanol: soluble
Auteur
Bayer
Température de stockage
2-8°C
Chaîne SMILES
COC(=O)C1=C(C)NC(C)=C(C1c2ccccc2[N+]([O-])=O)C(=O)OC
InChI
1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
Clé InChI
HYIMSNHJOBLJNT-UHFFFAOYSA-N
Informations sur le gène
human ... ADORA2A(135) , ADORA3(140) , CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779) , CACNA2D1(781) , CYP1A2(1544) , KCNH1(3756) , TTR(7276)
mouse ... Cacna1c(12288)
rat ... Adora1(29290) , Adora2a(25369) , Cacna1c(24239) , Cacna1d(29716) , Kcnj1(24521) , Kcnn4(65206) , Tbxas1(24886)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- to evaluate its effect on myenteric neuronal calcium current through R-type calcium channel in guinea pig small intestine[1]
- to evaluate the neuroprotective effect of L-type calcium channel blockers in cholinergic and dopaminergic neurons[2]
- to identify the effect of co-administration of nifedipine (anti-hypertensive drug) along with hypoglycemic drug on human umbilical vein cells (HUVECs)[3]
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Discover Bioactive Small Molecules for ADME/Tox
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
Are there any recommended dosages for Product N7634, Nifidipine, in vivo and in vitro?
1 answer-
Nifedipine is reported to inhibit Ca2+-sensitive K+ channels at 100 μM1. Doses for different animals have been reported2,3. In randomly growing cultures of aortic cells of rats, nifedipine at 10 μM inhibited cell proliferation. References: 1. Thomas-Young, R.J. et al. Biochim. Biophys. Acta, 1146, 81, (1993). 2. Drug Dosages In Laboratory Animals: A Handbook, 3rd ed., Telford Press. and 3. Borchard, R.E. et al. Drug Dosage in Laboratory Animals, A Handbook, Third Edition, p. 315, The Telford Press, (1990).
Helpful?
-
-
Are there any recommended conditions that Product N7634, Nifidipine, should be used in?
1 answer-
When exposed to daylight and certain wavelengths of artificial light, nifedipine readily converts to a nitrosophenylpyridine derivative. Exposure to ultra-violet light leads to the formation of a nitrophenylpyridine derivative. Therefore, USP recommends that assays be performed in the dark or under golden fluorescent or other low actinic light. Low actinic glassware should be used1.
Helpful?
-
-
What is the half life of Product N7634, Nifidipine, in vivo?
1 answer-
After administration by the mouth, the half-life is 2 to 5 hours 1. In plasma, about 92-98% binds to plasma proteins. Nifedipine is completely metabolized. About 70% of a dose is excreted in the urine in 24 hours as metabolites including 5-methoxycarbonyl-2,6-dimethyl-4-(2-nitrophenyl) pyridine-3-carboxylic acid; dimethyl 2,6-dimethyl-4-(2-nitrophenyl pyridine-3,5-dicarboxylate and 2-hydroxymethyl-5-methoxycarbonyl-6-methyl-4-(2-nitrophenyl) pyridine-3-carboxylic acid and its lactone derivative. Up to 15% of a dose is eliminated in the feces as metabolites in 4 days 2. References: 1. Martindale, 29th ed., pgs. 1509-1513. 2. Clarke's Isolation and Identification of Drugs., 2nd ed., p. 811.
Helpful?
-
-
What is the solubility of Product N7634, Nifidipine?
1 answer-
Nifedipine can be dissolved in DMSO at 50 mg/ml1. It is sparingly soluble in absolute ethanol2. Herembert, T. et al., dissolved nifedipine in absolute ethanol (no concentration reported); the maximum ethanol concentration in cultures was 0.2% without any effect of solvent on the cells3. Nifedipine is soluble (g/L, at 20°C) in the following solvents: acetone, 250; methylene chloride, 160; chloroform, 140; ethyl acetate, 50; methanol, 26; ethanol, 17.4 It is practically insoluble in water. The solubilities at 37°C in buffer solutions of different pH values are: pH 4, 0.0058 g/L; pH 7, 0.0056 g/L; pH 9.0, 0.0078 g/L; pH 13, 0.006 g/L1. References: 1. Ali, S.L., Analytical Profiles of Drug Substances, 18, 221, (1989). 2. Martindale, The Extra Pharmacopoeia, 30th ed., 374, (1993). and 3. Herembert, T. et al. Brit. J. Pharmacol., 114, 1703, (1995).
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique