K0133
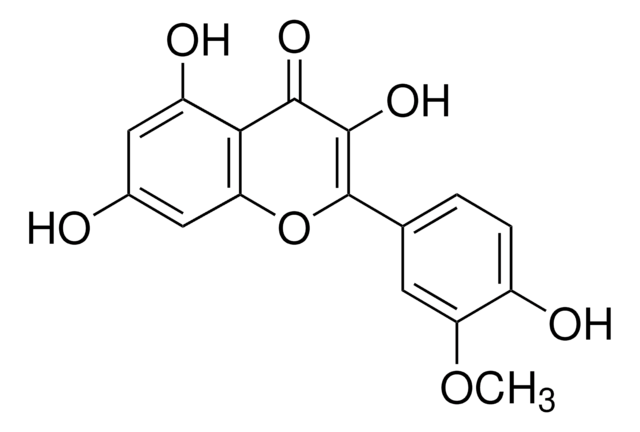
Kaempferol
≥90% (HPLC), powder
Synonyme(s) :
3,4′,5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, Robigenin
About This Item
Produits recommandés
Source biologique
synthetic
Pureté
≥90% (HPLC)
Forme
powder
Conditions de stockage
protect from light
Couleur
yellow
Pf
277 °C
Solubilité
ethanol: 20 mg/mL
DMSO: 50 mg/mL
Température de stockage
room temp
Chaîne SMILES
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
Clé InChI
IYRMWMYZSQPJKC-UHFFFAOYSA-N
Informations sur le gène
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- to check its potential effect as an antioxidant and neuroprotective agent against rotenone-induced Parkinson′s disease (PD) model in SH-S5Y5 cells
- to test its anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammatory injury in human aortic endothelial cells (HAECs)
- to study its apoptosis sensitizing effect on non-small cell lung cancer (NSCLC) cells by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2)
Actions biochimiques/physiologiques
Conditionnement
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique