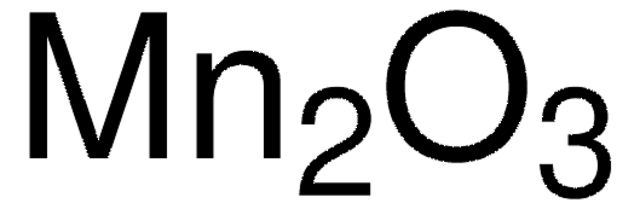310700
Manganese(IV) oxide
10 μm, reagent grade, ≥90%
Synonyme(s) :
Manganese dioxide
About This Item
Produits recommandés
Qualité
reagent grade
Pureté
≥90%
Forme
powder
Taille des particules
10 μm
Pf
535 °C (dec.) (lit.)
Chaîne SMILES
O=[Mn]=O
InChI
1S/Mn.2O
Clé InChI
NUJOXMJBOLGQSY-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- High-oxidation-state 3d metal complexes: Explores the catalytic properties of manganese(IV) oxide within high-oxidation-state complexes for advanced organic synthesis, demonstrating its critical role in accelerating chemical reactions and enhancing yield efficiencies, beneficial for pharmaceutical and chemical industries (Cheng J et al., 2018).
- Synthesis and properties of manganese complexes: Details the synthesis of new manganese complexes that demonstrate unique redox properties, useful for understanding electron transfer processes in various chemical and environmental contexts (Baffert C et al., 2002).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - STOT RE 2 Inhalation
Organes cibles
Brain
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
does not flash
Point d'éclair (°C)
does not flash
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique